Content Of Active Ingredient Test . The european medicines agency's scientific guidelines on the quality aspects of active substances help medicine developers. To summarize, the analysis results for the active component of tablets in a batch fall on a normal distribution. Testing of active pharmaceutical ingredients (apis) is a routine activity in many official medicines control laboratories (omcls) of the. Quality control tests of tablets or evaluation of tablets is a systematic determination of physical, chemical,. High performance (or ultra high performance) liquid chromatography (hplc or uhplc) gas chromatography (gc) titrations. The content uniformity test shows the distribution of the active content within the production batch. Ultraviolet light absorption (uv) infrared absorption (ir) atomic absorption (aa) The variability of the active ingredient derives from variability in: Nelson labs performs assay tests on active pharmaceutical ingredients (apis) and finished products, using techniques such as the following:
from eureka.patsnap.com
The variability of the active ingredient derives from variability in: Quality control tests of tablets or evaluation of tablets is a systematic determination of physical, chemical,. The european medicines agency's scientific guidelines on the quality aspects of active substances help medicine developers. Nelson labs performs assay tests on active pharmaceutical ingredients (apis) and finished products, using techniques such as the following: Testing of active pharmaceutical ingredients (apis) is a routine activity in many official medicines control laboratories (omcls) of the. High performance (or ultra high performance) liquid chromatography (hplc or uhplc) gas chromatography (gc) titrations. Ultraviolet light absorption (uv) infrared absorption (ir) atomic absorption (aa) The content uniformity test shows the distribution of the active content within the production batch. To summarize, the analysis results for the active component of tablets in a batch fall on a normal distribution.
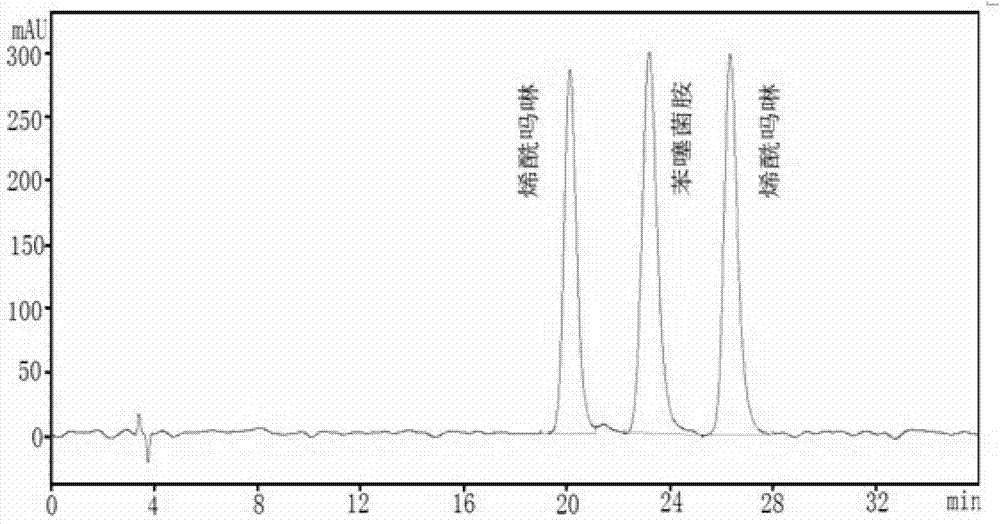
A method for analyzing the content of active ingredients in benthiazil
Content Of Active Ingredient Test Quality control tests of tablets or evaluation of tablets is a systematic determination of physical, chemical,. Testing of active pharmaceutical ingredients (apis) is a routine activity in many official medicines control laboratories (omcls) of the. Quality control tests of tablets or evaluation of tablets is a systematic determination of physical, chemical,. To summarize, the analysis results for the active component of tablets in a batch fall on a normal distribution. The content uniformity test shows the distribution of the active content within the production batch. The variability of the active ingredient derives from variability in: Ultraviolet light absorption (uv) infrared absorption (ir) atomic absorption (aa) Nelson labs performs assay tests on active pharmaceutical ingredients (apis) and finished products, using techniques such as the following: High performance (or ultra high performance) liquid chromatography (hplc or uhplc) gas chromatography (gc) titrations. The european medicines agency's scientific guidelines on the quality aspects of active substances help medicine developers.
From www.researchgate.net
Active ingredients, formulations, and manufacturers of herbicides used Content Of Active Ingredient Test The variability of the active ingredient derives from variability in: To summarize, the analysis results for the active component of tablets in a batch fall on a normal distribution. Ultraviolet light absorption (uv) infrared absorption (ir) atomic absorption (aa) The european medicines agency's scientific guidelines on the quality aspects of active substances help medicine developers. Testing of active pharmaceutical ingredients. Content Of Active Ingredient Test.
From slidetodoc.com
Quality control Quality Control Q C Definition Quality Content Of Active Ingredient Test To summarize, the analysis results for the active component of tablets in a batch fall on a normal distribution. Testing of active pharmaceutical ingredients (apis) is a routine activity in many official medicines control laboratories (omcls) of the. The content uniformity test shows the distribution of the active content within the production batch. Ultraviolet light absorption (uv) infrared absorption (ir). Content Of Active Ingredient Test.
From www.researchgate.net
Basic information of some active ingredients of ECXB Download Content Of Active Ingredient Test Nelson labs performs assay tests on active pharmaceutical ingredients (apis) and finished products, using techniques such as the following: High performance (or ultra high performance) liquid chromatography (hplc or uhplc) gas chromatography (gc) titrations. Quality control tests of tablets or evaluation of tablets is a systematic determination of physical, chemical,. The european medicines agency's scientific guidelines on the quality aspects. Content Of Active Ingredient Test.
From eureka.patsnap.com
Method for evaluating quality level through detection of contents of Content Of Active Ingredient Test Nelson labs performs assay tests on active pharmaceutical ingredients (apis) and finished products, using techniques such as the following: Ultraviolet light absorption (uv) infrared absorption (ir) atomic absorption (aa) The european medicines agency's scientific guidelines on the quality aspects of active substances help medicine developers. The content uniformity test shows the distribution of the active content within the production batch.. Content Of Active Ingredient Test.
From www.youtube.com
Sustainable active ingredients in cosmetic formulations OnlyTRAININGS Content Of Active Ingredient Test Nelson labs performs assay tests on active pharmaceutical ingredients (apis) and finished products, using techniques such as the following: The variability of the active ingredient derives from variability in: To summarize, the analysis results for the active component of tablets in a batch fall on a normal distribution. Ultraviolet light absorption (uv) infrared absorption (ir) atomic absorption (aa) The european. Content Of Active Ingredient Test.
From missalicecosmetics.com
Active Ingredients for Beginners Miss Alice Cosmetics Content Of Active Ingredient Test To summarize, the analysis results for the active component of tablets in a batch fall on a normal distribution. The variability of the active ingredient derives from variability in: Quality control tests of tablets or evaluation of tablets is a systematic determination of physical, chemical,. Nelson labs performs assay tests on active pharmaceutical ingredients (apis) and finished products, using techniques. Content Of Active Ingredient Test.
From www.researchgate.net
List of active ingredients tested for their residues Download Content Of Active Ingredient Test The european medicines agency's scientific guidelines on the quality aspects of active substances help medicine developers. The content uniformity test shows the distribution of the active content within the production batch. To summarize, the analysis results for the active component of tablets in a batch fall on a normal distribution. Quality control tests of tablets or evaluation of tablets is. Content Of Active Ingredient Test.
From 360researchpost.com
Understanding Active Pharmaceutical Ingredients (API) and Their Content Of Active Ingredient Test High performance (or ultra high performance) liquid chromatography (hplc or uhplc) gas chromatography (gc) titrations. Testing of active pharmaceutical ingredients (apis) is a routine activity in many official medicines control laboratories (omcls) of the. The european medicines agency's scientific guidelines on the quality aspects of active substances help medicine developers. Ultraviolet light absorption (uv) infrared absorption (ir) atomic absorption (aa). Content Of Active Ingredient Test.
From catalog.newsystemonline.com
GOJO® Purell® Surface Wipes Active Ingredient Test Strips New System Content Of Active Ingredient Test The content uniformity test shows the distribution of the active content within the production batch. To summarize, the analysis results for the active component of tablets in a batch fall on a normal distribution. The european medicines agency's scientific guidelines on the quality aspects of active substances help medicine developers. Ultraviolet light absorption (uv) infrared absorption (ir) atomic absorption (aa). Content Of Active Ingredient Test.
From www.youtube.com
What are the active ingredients? YouTube Content Of Active Ingredient Test The variability of the active ingredient derives from variability in: Testing of active pharmaceutical ingredients (apis) is a routine activity in many official medicines control laboratories (omcls) of the. Nelson labs performs assay tests on active pharmaceutical ingredients (apis) and finished products, using techniques such as the following: Quality control tests of tablets or evaluation of tablets is a systematic. Content Of Active Ingredient Test.
From www.pinterest.cl
Pin on Active Ingredients Content Of Active Ingredient Test The variability of the active ingredient derives from variability in: Nelson labs performs assay tests on active pharmaceutical ingredients (apis) and finished products, using techniques such as the following: The content uniformity test shows the distribution of the active content within the production batch. Quality control tests of tablets or evaluation of tablets is a systematic determination of physical, chemical,.. Content Of Active Ingredient Test.
From www.researchgate.net
Active ingredients and targets of QZF (YZ and TM) Download Scientific Content Of Active Ingredient Test The variability of the active ingredient derives from variability in: The european medicines agency's scientific guidelines on the quality aspects of active substances help medicine developers. Ultraviolet light absorption (uv) infrared absorption (ir) atomic absorption (aa) Quality control tests of tablets or evaluation of tablets is a systematic determination of physical, chemical,. High performance (or ultra high performance) liquid chromatography. Content Of Active Ingredient Test.
From www.mdpi.com
Extraction and Analysis of Plant Active Ingredients MDPI Books Content Of Active Ingredient Test Ultraviolet light absorption (uv) infrared absorption (ir) atomic absorption (aa) Quality control tests of tablets or evaluation of tablets is a systematic determination of physical, chemical,. The variability of the active ingredient derives from variability in: The european medicines agency's scientific guidelines on the quality aspects of active substances help medicine developers. Testing of active pharmaceutical ingredients (apis) is a. Content Of Active Ingredient Test.
From www.etundra.com
Purell 33416CTSTRP Surface Spray Active Ingredient Test Strips Content Of Active Ingredient Test Testing of active pharmaceutical ingredients (apis) is a routine activity in many official medicines control laboratories (omcls) of the. The variability of the active ingredient derives from variability in: To summarize, the analysis results for the active component of tablets in a batch fall on a normal distribution. The european medicines agency's scientific guidelines on the quality aspects of active. Content Of Active Ingredient Test.
From www.webstaurantstore.com
Purell® 33416CTSTRP Active Ingredient Concentration Test Strip 6/Pack Content Of Active Ingredient Test The variability of the active ingredient derives from variability in: High performance (or ultra high performance) liquid chromatography (hplc or uhplc) gas chromatography (gc) titrations. To summarize, the analysis results for the active component of tablets in a batch fall on a normal distribution. Ultraviolet light absorption (uv) infrared absorption (ir) atomic absorption (aa) Testing of active pharmaceutical ingredients (apis). Content Of Active Ingredient Test.
From www.researchgate.net
List of active pharmaceutical ingredients (APIs) selected for the study Content Of Active Ingredient Test Nelson labs performs assay tests on active pharmaceutical ingredients (apis) and finished products, using techniques such as the following: To summarize, the analysis results for the active component of tablets in a batch fall on a normal distribution. Ultraviolet light absorption (uv) infrared absorption (ir) atomic absorption (aa) The content uniformity test shows the distribution of the active content within. Content Of Active Ingredient Test.
From www.researchgate.net
Results from active ingredients release tests highlighting the Content Of Active Ingredient Test Nelson labs performs assay tests on active pharmaceutical ingredients (apis) and finished products, using techniques such as the following: Testing of active pharmaceutical ingredients (apis) is a routine activity in many official medicines control laboratories (omcls) of the. High performance (or ultra high performance) liquid chromatography (hplc or uhplc) gas chromatography (gc) titrations. The european medicines agency's scientific guidelines on. Content Of Active Ingredient Test.
From www.pdfprof.com
check ingredients in cosmetics app uk Content Of Active Ingredient Test Testing of active pharmaceutical ingredients (apis) is a routine activity in many official medicines control laboratories (omcls) of the. The variability of the active ingredient derives from variability in: Ultraviolet light absorption (uv) infrared absorption (ir) atomic absorption (aa) To summarize, the analysis results for the active component of tablets in a batch fall on a normal distribution. Quality control. Content Of Active Ingredient Test.
From www.researchgate.net
Herbicide program, active ingredient, rate, and timings for Content Of Active Ingredient Test The variability of the active ingredient derives from variability in: To summarize, the analysis results for the active component of tablets in a batch fall on a normal distribution. The content uniformity test shows the distribution of the active content within the production batch. The european medicines agency's scientific guidelines on the quality aspects of active substances help medicine developers.. Content Of Active Ingredient Test.
From www.researchgate.net
the most important active ingredients included Download Table Content Of Active Ingredient Test The variability of the active ingredient derives from variability in: Ultraviolet light absorption (uv) infrared absorption (ir) atomic absorption (aa) Testing of active pharmaceutical ingredients (apis) is a routine activity in many official medicines control laboratories (omcls) of the. To summarize, the analysis results for the active component of tablets in a batch fall on a normal distribution. The european. Content Of Active Ingredient Test.
From eureka.patsnap.com
A method for analyzing the content of active ingredients in benthiazil Content Of Active Ingredient Test To summarize, the analysis results for the active component of tablets in a batch fall on a normal distribution. The content uniformity test shows the distribution of the active content within the production batch. High performance (or ultra high performance) liquid chromatography (hplc or uhplc) gas chromatography (gc) titrations. The variability of the active ingredient derives from variability in: The. Content Of Active Ingredient Test.
From twitter.com
EEF on Twitter "Active ingredients this summary supports the 'A Content Of Active Ingredient Test Nelson labs performs assay tests on active pharmaceutical ingredients (apis) and finished products, using techniques such as the following: Testing of active pharmaceutical ingredients (apis) is a routine activity in many official medicines control laboratories (omcls) of the. To summarize, the analysis results for the active component of tablets in a batch fall on a normal distribution. The european medicines. Content Of Active Ingredient Test.
From blog.gopicky.com
Active vs. Inactive Ingredients Picky The KBeauty Hot Place Content Of Active Ingredient Test The variability of the active ingredient derives from variability in: The european medicines agency's scientific guidelines on the quality aspects of active substances help medicine developers. Ultraviolet light absorption (uv) infrared absorption (ir) atomic absorption (aa) The content uniformity test shows the distribution of the active content within the production batch. Nelson labs performs assay tests on active pharmaceutical ingredients. Content Of Active Ingredient Test.
From www.webstaurantstore.com
Purell® 33416CTSTRP Active Ingredient Concentration Test Strip 6/Pack Content Of Active Ingredient Test The variability of the active ingredient derives from variability in: Testing of active pharmaceutical ingredients (apis) is a routine activity in many official medicines control laboratories (omcls) of the. To summarize, the analysis results for the active component of tablets in a batch fall on a normal distribution. High performance (or ultra high performance) liquid chromatography (hplc or uhplc) gas. Content Of Active Ingredient Test.
From www.researchgate.net
DETERMINATIONS OF ACTIVE INGREDIENTS IN TABLETS Download Scientific Content Of Active Ingredient Test Ultraviolet light absorption (uv) infrared absorption (ir) atomic absorption (aa) High performance (or ultra high performance) liquid chromatography (hplc or uhplc) gas chromatography (gc) titrations. Quality control tests of tablets or evaluation of tablets is a systematic determination of physical, chemical,. To summarize, the analysis results for the active component of tablets in a batch fall on a normal distribution.. Content Of Active Ingredient Test.
From irenebeautyandmore.com
What are Active Ingredients in Cosmetics? » Irene Content Of Active Ingredient Test The european medicines agency's scientific guidelines on the quality aspects of active substances help medicine developers. Quality control tests of tablets or evaluation of tablets is a systematic determination of physical, chemical,. Nelson labs performs assay tests on active pharmaceutical ingredients (apis) and finished products, using techniques such as the following: The variability of the active ingredient derives from variability. Content Of Active Ingredient Test.
From www.slideserve.com
PPT Solid Dosage Forms (Tablets) PowerPoint Presentation, free Content Of Active Ingredient Test Quality control tests of tablets or evaluation of tablets is a systematic determination of physical, chemical,. The variability of the active ingredient derives from variability in: The content uniformity test shows the distribution of the active content within the production batch. Ultraviolet light absorption (uv) infrared absorption (ir) atomic absorption (aa) Testing of active pharmaceutical ingredients (apis) is a routine. Content Of Active Ingredient Test.
From slideplayer.com
Quality control Lecture ppt download Content Of Active Ingredient Test Ultraviolet light absorption (uv) infrared absorption (ir) atomic absorption (aa) To summarize, the analysis results for the active component of tablets in a batch fall on a normal distribution. High performance (or ultra high performance) liquid chromatography (hplc or uhplc) gas chromatography (gc) titrations. The european medicines agency's scientific guidelines on the quality aspects of active substances help medicine developers.. Content Of Active Ingredient Test.
From www.splyco.com
Purell® 33416CTSTRP Active Ingredient Concentration Test Strip 6 Content Of Active Ingredient Test Ultraviolet light absorption (uv) infrared absorption (ir) atomic absorption (aa) Testing of active pharmaceutical ingredients (apis) is a routine activity in many official medicines control laboratories (omcls) of the. Nelson labs performs assay tests on active pharmaceutical ingredients (apis) and finished products, using techniques such as the following: Quality control tests of tablets or evaluation of tablets is a systematic. Content Of Active Ingredient Test.
From www.researchgate.net
The average content of active ingredients in different commercial Content Of Active Ingredient Test High performance (or ultra high performance) liquid chromatography (hplc or uhplc) gas chromatography (gc) titrations. To summarize, the analysis results for the active component of tablets in a batch fall on a normal distribution. Nelson labs performs assay tests on active pharmaceutical ingredients (apis) and finished products, using techniques such as the following: The european medicines agency's scientific guidelines on. Content Of Active Ingredient Test.
From xcelpros.com
Active Ingredient & Batch Balancing in Batch Production Content Of Active Ingredient Test High performance (or ultra high performance) liquid chromatography (hplc or uhplc) gas chromatography (gc) titrations. The content uniformity test shows the distribution of the active content within the production batch. To summarize, the analysis results for the active component of tablets in a batch fall on a normal distribution. Testing of active pharmaceutical ingredients (apis) is a routine activity in. Content Of Active Ingredient Test.
From www.researchgate.net
Screening and network construction of active ingredients. (a) Active Content Of Active Ingredient Test The variability of the active ingredient derives from variability in: High performance (or ultra high performance) liquid chromatography (hplc or uhplc) gas chromatography (gc) titrations. The content uniformity test shows the distribution of the active content within the production batch. To summarize, the analysis results for the active component of tablets in a batch fall on a normal distribution. Ultraviolet. Content Of Active Ingredient Test.
From www.numerade.com
The process of producing painreliever tablets yields tablets with Content Of Active Ingredient Test Nelson labs performs assay tests on active pharmaceutical ingredients (apis) and finished products, using techniques such as the following: To summarize, the analysis results for the active component of tablets in a batch fall on a normal distribution. The european medicines agency's scientific guidelines on the quality aspects of active substances help medicine developers. The variability of the active ingredient. Content Of Active Ingredient Test.
From www.youtube.com
Determining Quantity of Active Ingredient from Diluted Portion YouTube Content Of Active Ingredient Test Testing of active pharmaceutical ingredients (apis) is a routine activity in many official medicines control laboratories (omcls) of the. High performance (or ultra high performance) liquid chromatography (hplc or uhplc) gas chromatography (gc) titrations. The content uniformity test shows the distribution of the active content within the production batch. Quality control tests of tablets or evaluation of tablets is a. Content Of Active Ingredient Test.
From catalog.newsystemonline.com
GOJO® Purell® Surface Wipes Active Ingredient Test Strips New System Content Of Active Ingredient Test To summarize, the analysis results for the active component of tablets in a batch fall on a normal distribution. High performance (or ultra high performance) liquid chromatography (hplc or uhplc) gas chromatography (gc) titrations. Quality control tests of tablets or evaluation of tablets is a systematic determination of physical, chemical,. The content uniformity test shows the distribution of the active. Content Of Active Ingredient Test.