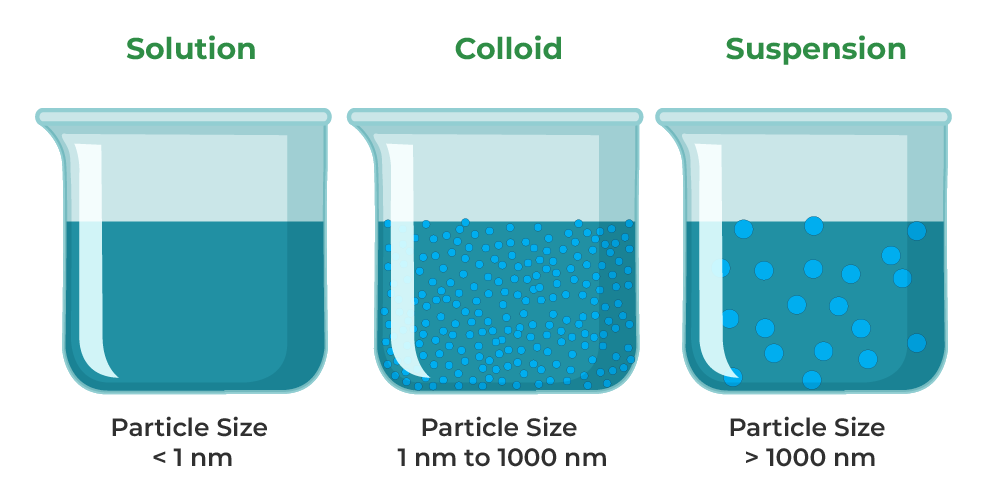
What Is Solution Suspension And Colloid . A solution may be colored, but it is transparent, the molecules or ions are. Suspensions and colloids are two common types of mixtures whose properties are in many ways intermediate between those of homogeneous and heterogeneous mixtures. A colloid is a type of mixture where small particles of one substance are suspended and distributed throughout a continuous medium. Learn what a colloid is in chemistry. Solutions exhibit completely different behavior from suspensions. See how it differs from a solution or suspension. Get the definition and colloid examples. A colloid is a heterogeneous mixture in which the dispersed particles are intermediate in size between those of a solution and a. Suspensions and colloids differ from solution in that a solution is homogeneous, contains dissolved particles, and is translucent. A colloid is a mixture in which one of the soluble or insoluble particles is microscopically dispersed throughout the other.
from www.geeksforgeeks.org
Suspensions and colloids differ from solution in that a solution is homogeneous, contains dissolved particles, and is translucent. Solutions exhibit completely different behavior from suspensions. Learn what a colloid is in chemistry. A solution may be colored, but it is transparent, the molecules or ions are. Get the definition and colloid examples. A colloid is a type of mixture where small particles of one substance are suspended and distributed throughout a continuous medium. A colloid is a heterogeneous mixture in which the dispersed particles are intermediate in size between those of a solution and a. See how it differs from a solution or suspension. Suspensions and colloids are two common types of mixtures whose properties are in many ways intermediate between those of homogeneous and heterogeneous mixtures. A colloid is a mixture in which one of the soluble or insoluble particles is microscopically dispersed throughout the other.
Suspension(Chemistry) Definition, Properties, Examples, and FAQs
What Is Solution Suspension And Colloid A solution may be colored, but it is transparent, the molecules or ions are. A colloid is a type of mixture where small particles of one substance are suspended and distributed throughout a continuous medium. Suspensions and colloids are two common types of mixtures whose properties are in many ways intermediate between those of homogeneous and heterogeneous mixtures. Solutions exhibit completely different behavior from suspensions. Get the definition and colloid examples. A colloid is a mixture in which one of the soluble or insoluble particles is microscopically dispersed throughout the other. Suspensions and colloids differ from solution in that a solution is homogeneous, contains dissolved particles, and is translucent. Learn what a colloid is in chemistry. See how it differs from a solution or suspension. A solution may be colored, but it is transparent, the molecules or ions are. A colloid is a heterogeneous mixture in which the dispersed particles are intermediate in size between those of a solution and a.
From www.youtube.com
Solutions Suspensions and Colloids Part 1/1 English Class 9 YouTube What Is Solution Suspension And Colloid See how it differs from a solution or suspension. Suspensions and colloids are two common types of mixtures whose properties are in many ways intermediate between those of homogeneous and heterogeneous mixtures. Solutions exhibit completely different behavior from suspensions. A colloid is a type of mixture where small particles of one substance are suspended and distributed throughout a continuous medium.. What Is Solution Suspension And Colloid.
From www.vecteezy.com
Different dispersion system, true and colloidal solution and suspension What Is Solution Suspension And Colloid See how it differs from a solution or suspension. Suspensions and colloids are two common types of mixtures whose properties are in many ways intermediate between those of homogeneous and heterogeneous mixtures. Suspensions and colloids differ from solution in that a solution is homogeneous, contains dissolved particles, and is translucent. A colloid is a heterogeneous mixture in which the dispersed. What Is Solution Suspension And Colloid.
From byjus.com
Suspensions (Chemistry) Definition, Properties, Examples with Videos What Is Solution Suspension And Colloid Solutions exhibit completely different behavior from suspensions. A colloid is a type of mixture where small particles of one substance are suspended and distributed throughout a continuous medium. A colloid is a mixture in which one of the soluble or insoluble particles is microscopically dispersed throughout the other. A solution may be colored, but it is transparent, the molecules or. What Is Solution Suspension And Colloid.
From in.pinterest.com
Solutions, suspensions, and colloids Suspension mixture, Body diagram What Is Solution Suspension And Colloid See how it differs from a solution or suspension. Suspensions and colloids are two common types of mixtures whose properties are in many ways intermediate between those of homogeneous and heterogeneous mixtures. Suspensions and colloids differ from solution in that a solution is homogeneous, contains dissolved particles, and is translucent. Learn what a colloid is in chemistry. A solution may. What Is Solution Suspension And Colloid.
From brainly.in
draw diagram illustrate true solutions suspension and colloidal What Is Solution Suspension And Colloid Learn what a colloid is in chemistry. Suspensions and colloids differ from solution in that a solution is homogeneous, contains dissolved particles, and is translucent. Solutions exhibit completely different behavior from suspensions. A colloid is a heterogeneous mixture in which the dispersed particles are intermediate in size between those of a solution and a. A solution may be colored, but. What Is Solution Suspension And Colloid.
From www.youtube.com
Solution Suspension Colloid YouTube What Is Solution Suspension And Colloid A colloid is a heterogeneous mixture in which the dispersed particles are intermediate in size between those of a solution and a. A colloid is a type of mixture where small particles of one substance are suspended and distributed throughout a continuous medium. See how it differs from a solution or suspension. Learn what a colloid is in chemistry. Suspensions. What Is Solution Suspension And Colloid.
From www.majordifferences.com
Difference Between True Solutions, Colloidal solution and Suspension What Is Solution Suspension And Colloid Get the definition and colloid examples. Solutions exhibit completely different behavior from suspensions. A colloid is a mixture in which one of the soluble or insoluble particles is microscopically dispersed throughout the other. A colloid is a type of mixture where small particles of one substance are suspended and distributed throughout a continuous medium. Suspensions and colloids are two common. What Is Solution Suspension And Colloid.
From www.youtube.com
SCIENCE 6 SUSPENSION AND COLLOID YouTube What Is Solution Suspension And Colloid See how it differs from a solution or suspension. A colloid is a mixture in which one of the soluble or insoluble particles is microscopically dispersed throughout the other. Suspensions and colloids differ from solution in that a solution is homogeneous, contains dissolved particles, and is translucent. Suspensions and colloids are two common types of mixtures whose properties are in. What Is Solution Suspension And Colloid.
From plantlet.org
Mixture Types Solution, Suspension, Colloids & Others Plantlet What Is Solution Suspension And Colloid A colloid is a type of mixture where small particles of one substance are suspended and distributed throughout a continuous medium. Suspensions and colloids differ from solution in that a solution is homogeneous, contains dissolved particles, and is translucent. Learn what a colloid is in chemistry. A colloid is a heterogeneous mixture in which the dispersed particles are intermediate in. What Is Solution Suspension And Colloid.
From sciencenotes.org
What Is a Colloid? Definition and Examples What Is Solution Suspension And Colloid Suspensions and colloids are two common types of mixtures whose properties are in many ways intermediate between those of homogeneous and heterogeneous mixtures. A colloid is a mixture in which one of the soluble or insoluble particles is microscopically dispersed throughout the other. Get the definition and colloid examples. Suspensions and colloids differ from solution in that a solution is. What Is Solution Suspension And Colloid.
From quizizz.com
Solution, Colloid and Suspension Science Quizizz What Is Solution Suspension And Colloid A colloid is a heterogeneous mixture in which the dispersed particles are intermediate in size between those of a solution and a. See how it differs from a solution or suspension. Get the definition and colloid examples. Solutions exhibit completely different behavior from suspensions. Suspensions and colloids are two common types of mixtures whose properties are in many ways intermediate. What Is Solution Suspension And Colloid.
From www.youtube.com
Science 6 Q1 Week 2 Solution, Suspension, Colloid YouTube What Is Solution Suspension And Colloid A colloid is a heterogeneous mixture in which the dispersed particles are intermediate in size between those of a solution and a. Get the definition and colloid examples. Learn what a colloid is in chemistry. Suspensions and colloids differ from solution in that a solution is homogeneous, contains dissolved particles, and is translucent. A colloid is a type of mixture. What Is Solution Suspension And Colloid.
From www.slideserve.com
PPT CH110 Chapter 8 Solutions PowerPoint Presentation, free download What Is Solution Suspension And Colloid A solution may be colored, but it is transparent, the molecules or ions are. Suspensions and colloids are two common types of mixtures whose properties are in many ways intermediate between those of homogeneous and heterogeneous mixtures. A colloid is a type of mixture where small particles of one substance are suspended and distributed throughout a continuous medium. A colloid. What Is Solution Suspension And Colloid.
From www.myshared.ru
Презентация на тему "Colloidal systems. Classes of solution True What Is Solution Suspension And Colloid Learn what a colloid is in chemistry. A colloid is a mixture in which one of the soluble or insoluble particles is microscopically dispersed throughout the other. Get the definition and colloid examples. Solutions exhibit completely different behavior from suspensions. A colloid is a heterogeneous mixture in which the dispersed particles are intermediate in size between those of a solution. What Is Solution Suspension And Colloid.
From www.differencebetween.com
Difference Between Suspension and Colloid Compare the Difference What Is Solution Suspension And Colloid A colloid is a type of mixture where small particles of one substance are suspended and distributed throughout a continuous medium. Suspensions and colloids differ from solution in that a solution is homogeneous, contains dissolved particles, and is translucent. See how it differs from a solution or suspension. Suspensions and colloids are two common types of mixtures whose properties are. What Is Solution Suspension And Colloid.
From www.slideserve.com
PPT Matter Properties & Change PowerPoint Presentation, free What Is Solution Suspension And Colloid Suspensions and colloids differ from solution in that a solution is homogeneous, contains dissolved particles, and is translucent. A colloid is a mixture in which one of the soluble or insoluble particles is microscopically dispersed throughout the other. Suspensions and colloids are two common types of mixtures whose properties are in many ways intermediate between those of homogeneous and heterogeneous. What Is Solution Suspension And Colloid.
From www.youtube.com
Solution, Suspension and Colloid aumsum kids education science What Is Solution Suspension And Colloid Suspensions and colloids differ from solution in that a solution is homogeneous, contains dissolved particles, and is translucent. Solutions exhibit completely different behavior from suspensions. See how it differs from a solution or suspension. Learn what a colloid is in chemistry. A colloid is a mixture in which one of the soluble or insoluble particles is microscopically dispersed throughout the. What Is Solution Suspension And Colloid.
From www.aakash.ac.in
Colloidal Solution Definition, Classification, Examples & Preparation What Is Solution Suspension And Colloid See how it differs from a solution or suspension. A colloid is a mixture in which one of the soluble or insoluble particles is microscopically dispersed throughout the other. Solutions exhibit completely different behavior from suspensions. A colloid is a heterogeneous mixture in which the dispersed particles are intermediate in size between those of a solution and a. Get the. What Is Solution Suspension And Colloid.
From www.geeksforgeeks.org
Suspension(Chemistry) Definition, Properties, Examples, and FAQs What Is Solution Suspension And Colloid A solution may be colored, but it is transparent, the molecules or ions are. Suspensions and colloids are two common types of mixtures whose properties are in many ways intermediate between those of homogeneous and heterogeneous mixtures. A colloid is a heterogeneous mixture in which the dispersed particles are intermediate in size between those of a solution and a. Solutions. What Is Solution Suspension And Colloid.
From byjus.com
Suspensions & Colloids Difference Between Colloid & SuspensionByju's What Is Solution Suspension And Colloid See how it differs from a solution or suspension. A solution may be colored, but it is transparent, the molecules or ions are. Get the definition and colloid examples. A colloid is a heterogeneous mixture in which the dispersed particles are intermediate in size between those of a solution and a. Learn what a colloid is in chemistry. A colloid. What Is Solution Suspension And Colloid.
From slideplayer.com
Solutions, Colloids, and Suspensions ppt download What Is Solution Suspension And Colloid Solutions exhibit completely different behavior from suspensions. A colloid is a type of mixture where small particles of one substance are suspended and distributed throughout a continuous medium. Learn what a colloid is in chemistry. Get the definition and colloid examples. A colloid is a heterogeneous mixture in which the dispersed particles are intermediate in size between those of a. What Is Solution Suspension And Colloid.
From slideplayer.com
Solutions, Colloids, and Suspensions ppt download What Is Solution Suspension And Colloid Learn what a colloid is in chemistry. A solution may be colored, but it is transparent, the molecules or ions are. Get the definition and colloid examples. Solutions exhibit completely different behavior from suspensions. A colloid is a mixture in which one of the soluble or insoluble particles is microscopically dispersed throughout the other. See how it differs from a. What Is Solution Suspension And Colloid.
From www.youtube.com
Colloids, Solutions & Suspensions YouTube What Is Solution Suspension And Colloid See how it differs from a solution or suspension. Learn what a colloid is in chemistry. A colloid is a heterogeneous mixture in which the dispersed particles are intermediate in size between those of a solution and a. Solutions exhibit completely different behavior from suspensions. A colloid is a mixture in which one of the soluble or insoluble particles is. What Is Solution Suspension And Colloid.
From www.slideserve.com
PPT Suspensions and Colloids PowerPoint Presentation, free download What Is Solution Suspension And Colloid Learn what a colloid is in chemistry. A colloid is a heterogeneous mixture in which the dispersed particles are intermediate in size between those of a solution and a. Suspensions and colloids differ from solution in that a solution is homogeneous, contains dissolved particles, and is translucent. A colloid is a type of mixture where small particles of one substance. What Is Solution Suspension And Colloid.
From www.slideserve.com
PPT Solution, Suspensions and Colloids PowerPoint Presentation, free What Is Solution Suspension And Colloid A colloid is a heterogeneous mixture in which the dispersed particles are intermediate in size between those of a solution and a. Suspensions and colloids are two common types of mixtures whose properties are in many ways intermediate between those of homogeneous and heterogeneous mixtures. Learn what a colloid is in chemistry. A solution may be colored, but it is. What Is Solution Suspension And Colloid.
From slideplayer.com
Solutions, Colloids, and Suspensions ppt download What Is Solution Suspension And Colloid See how it differs from a solution or suspension. A colloid is a type of mixture where small particles of one substance are suspended and distributed throughout a continuous medium. A colloid is a mixture in which one of the soluble or insoluble particles is microscopically dispersed throughout the other. A colloid is a heterogeneous mixture in which the dispersed. What Is Solution Suspension And Colloid.
From brainly.in
How are colloids and suspensions different from solutions ? Brainly.in What Is Solution Suspension And Colloid Suspensions and colloids differ from solution in that a solution is homogeneous, contains dissolved particles, and is translucent. A colloid is a type of mixture where small particles of one substance are suspended and distributed throughout a continuous medium. Get the definition and colloid examples. Solutions exhibit completely different behavior from suspensions. A solution may be colored, but it is. What Is Solution Suspension And Colloid.
From 88guru.com
True solution, Suspension, Colloidal Solutions 88Guru What Is Solution Suspension And Colloid See how it differs from a solution or suspension. A colloid is a heterogeneous mixture in which the dispersed particles are intermediate in size between those of a solution and a. Suspensions and colloids differ from solution in that a solution is homogeneous, contains dissolved particles, and is translucent. A colloid is a type of mixture where small particles of. What Is Solution Suspension And Colloid.
From www.slideserve.com
PPT Chapter 8 Solutions PowerPoint Presentation, free download ID What Is Solution Suspension And Colloid A solution may be colored, but it is transparent, the molecules or ions are. Solutions exhibit completely different behavior from suspensions. A colloid is a type of mixture where small particles of one substance are suspended and distributed throughout a continuous medium. A colloid is a mixture in which one of the soluble or insoluble particles is microscopically dispersed throughout. What Is Solution Suspension And Colloid.
From www.slideserve.com
PPT Chapter 12 SOLUTIONS PowerPoint Presentation, free download ID What Is Solution Suspension And Colloid Suspensions and colloids are two common types of mixtures whose properties are in many ways intermediate between those of homogeneous and heterogeneous mixtures. See how it differs from a solution or suspension. A colloid is a heterogeneous mixture in which the dispersed particles are intermediate in size between those of a solution and a. Solutions exhibit completely different behavior from. What Is Solution Suspension And Colloid.
From www.youtube.com
What is solution, Suspension and Colloid? Science Composition of What Is Solution Suspension And Colloid A solution may be colored, but it is transparent, the molecules or ions are. See how it differs from a solution or suspension. A colloid is a heterogeneous mixture in which the dispersed particles are intermediate in size between those of a solution and a. Get the definition and colloid examples. Learn what a colloid is in chemistry. Suspensions and. What Is Solution Suspension And Colloid.
From www.slideserve.com
PPT Suspensions and Colloids PowerPoint Presentation, free download What Is Solution Suspension And Colloid Get the definition and colloid examples. See how it differs from a solution or suspension. A colloid is a mixture in which one of the soluble or insoluble particles is microscopically dispersed throughout the other. Solutions exhibit completely different behavior from suspensions. A colloid is a heterogeneous mixture in which the dispersed particles are intermediate in size between those of. What Is Solution Suspension And Colloid.
From www.youtube.com
Solution colloids n suspension class 9 by neelam dwivedi YouTube What Is Solution Suspension And Colloid Solutions exhibit completely different behavior from suspensions. See how it differs from a solution or suspension. Suspensions and colloids are two common types of mixtures whose properties are in many ways intermediate between those of homogeneous and heterogeneous mixtures. Learn what a colloid is in chemistry. A colloid is a heterogeneous mixture in which the dispersed particles are intermediate in. What Is Solution Suspension And Colloid.
From pt.slideshare.net
Solutions, suspensions, and colloids What Is Solution Suspension And Colloid Get the definition and colloid examples. A colloid is a mixture in which one of the soluble or insoluble particles is microscopically dispersed throughout the other. A colloid is a type of mixture where small particles of one substance are suspended and distributed throughout a continuous medium. A solution may be colored, but it is transparent, the molecules or ions. What Is Solution Suspension And Colloid.
From www.vecteezy.com
True Solution, Colloid solution and Suspension three different types of What Is Solution Suspension And Colloid Suspensions and colloids differ from solution in that a solution is homogeneous, contains dissolved particles, and is translucent. See how it differs from a solution or suspension. A solution may be colored, but it is transparent, the molecules or ions are. Learn what a colloid is in chemistry. A colloid is a mixture in which one of the soluble or. What Is Solution Suspension And Colloid.