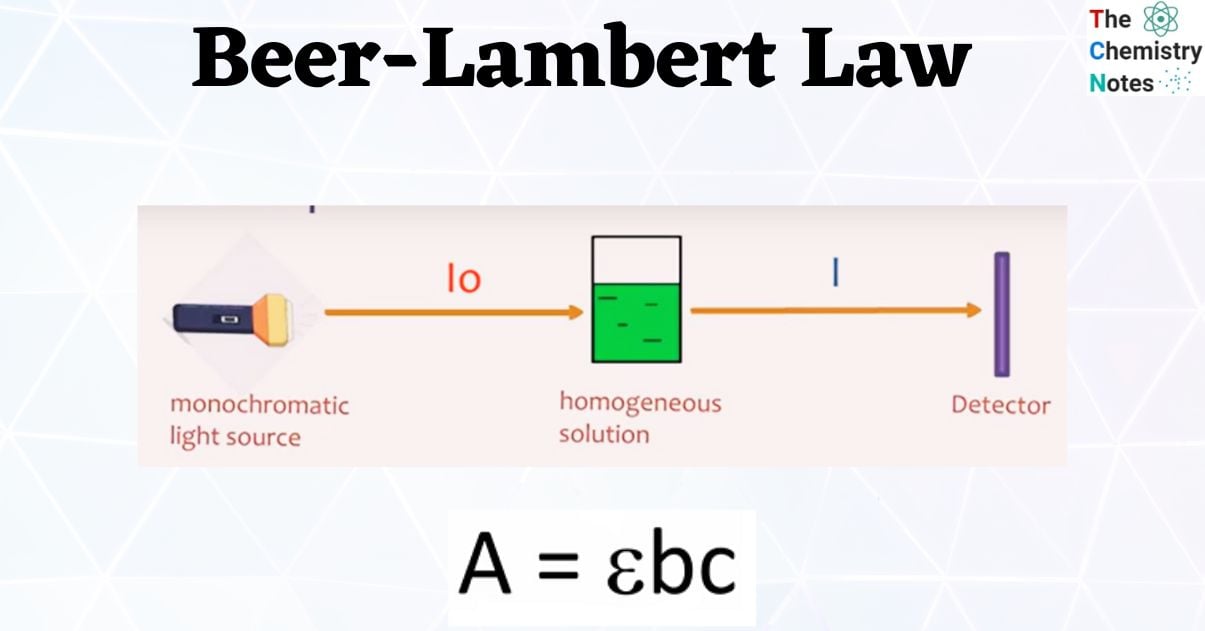
Beer's Law In Colorimetry . The colorimeter is usually used to measure the concentration of a. It refers to a device which helps specific solutions to absorb a particular wavelength of light. Determining the concentration of a solution. Beer's law is an equation that relates light's attenuation to a material's properties. Ensate for the natural color loss of food during storage. In this equation a is. Along with operating the instruments, beer's law also involves calculations to actually figure out the concentration of a solution from the. A = abc, where a is the absorbance of the solution, a is the molar. This relationship is known as beer’s law and is given by the equation: These factors and assumptions can be summarized as beer's law and written as the equation, a = abc. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following.
from scienceinfo.com
A = abc, where a is the absorbance of the solution, a is the molar. Beer's law is an equation that relates light's attenuation to a material's properties. Determining the concentration of a solution. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following. The colorimeter is usually used to measure the concentration of a. It refers to a device which helps specific solutions to absorb a particular wavelength of light. These factors and assumptions can be summarized as beer's law and written as the equation, a = abc. In this equation a is. This relationship is known as beer’s law and is given by the equation: Ensate for the natural color loss of food during storage.
BeerLambert Law Statement, Derivation, Applications, Limitations
Beer's Law In Colorimetry The colorimeter is usually used to measure the concentration of a. These factors and assumptions can be summarized as beer's law and written as the equation, a = abc. The colorimeter is usually used to measure the concentration of a. In this equation a is. This relationship is known as beer’s law and is given by the equation: Beer's law is an equation that relates light's attenuation to a material's properties. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following. Ensate for the natural color loss of food during storage. It refers to a device which helps specific solutions to absorb a particular wavelength of light. Along with operating the instruments, beer's law also involves calculations to actually figure out the concentration of a solution from the. A = abc, where a is the absorbance of the solution, a is the molar. Determining the concentration of a solution.
From www.slideserve.com
PPT Beer’s Law & Colorimetry PowerPoint Presentation, free download Beer's Law In Colorimetry The colorimeter is usually used to measure the concentration of a. Beer's law is an equation that relates light's attenuation to a material's properties. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following. A = abc, where a is the absorbance of the solution, a is the molar. Determining. Beer's Law In Colorimetry.
From www.slideserve.com
PPT Beer’s Law & Colorimetry PowerPoint Presentation, free download Beer's Law In Colorimetry Along with operating the instruments, beer's law also involves calculations to actually figure out the concentration of a solution from the. It refers to a device which helps specific solutions to absorb a particular wavelength of light. Determining the concentration of a solution. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can. Beer's Law In Colorimetry.
From www.slideserve.com
PPT Beer’s Law & Colorimetry PowerPoint Presentation, free download Beer's Law In Colorimetry These factors and assumptions can be summarized as beer's law and written as the equation, a = abc. In this equation a is. It refers to a device which helps specific solutions to absorb a particular wavelength of light. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following. This. Beer's Law In Colorimetry.
From www.slideserve.com
PPT Beer’s Law & Colorimetry PowerPoint Presentation, free download Beer's Law In Colorimetry These factors and assumptions can be summarized as beer's law and written as the equation, a = abc. Beer's law is an equation that relates light's attenuation to a material's properties. A = abc, where a is the absorbance of the solution, a is the molar. In this equation a is. Since the concentration, path length and molar absorptivity are. Beer's Law In Colorimetry.
From www.youtube.com
Spectroscopy Beer Lambert's Law YouTube Beer's Law In Colorimetry Along with operating the instruments, beer's law also involves calculations to actually figure out the concentration of a solution from the. This relationship is known as beer’s law and is given by the equation: The colorimeter is usually used to measure the concentration of a. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance,. Beer's Law In Colorimetry.
From www.youtube.com
Beer Lambert's Law, Absorbance & Transmittance Spectrophotometry Beer's Law In Colorimetry These factors and assumptions can be summarized as beer's law and written as the equation, a = abc. This relationship is known as beer’s law and is given by the equation: The colorimeter is usually used to measure the concentration of a. A = abc, where a is the absorbance of the solution, a is the molar. Since the concentration,. Beer's Law In Colorimetry.
From www.youtube.com
Introduction to Colorimetry Verify LambertBeer Law Experimental Beer's Law In Colorimetry Along with operating the instruments, beer's law also involves calculations to actually figure out the concentration of a solution from the. The colorimeter is usually used to measure the concentration of a. Beer's law is an equation that relates light's attenuation to a material's properties. A = abc, where a is the absorbance of the solution, a is the molar.. Beer's Law In Colorimetry.
From www.youtube.com
Colorimetry Beer's law Lambert's law biochemistry YouTube Beer's Law In Colorimetry A = abc, where a is the absorbance of the solution, a is the molar. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following. Along with operating the instruments, beer's law also involves calculations to actually figure out the concentration of a solution from the. This relationship is known. Beer's Law In Colorimetry.
From edusanjalbiochemist.blogspot.com
Biochemistry Class notes Photometry Principle, applications and types Beer's Law In Colorimetry Beer's law is an equation that relates light's attenuation to a material's properties. It refers to a device which helps specific solutions to absorb a particular wavelength of light. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following. This relationship is known as beer’s law and is given by. Beer's Law In Colorimetry.
From www.slideserve.com
PPT Beer’s Law & Colorimetry PowerPoint Presentation, free download Beer's Law In Colorimetry It refers to a device which helps specific solutions to absorb a particular wavelength of light. Determining the concentration of a solution. This relationship is known as beer’s law and is given by the equation: A = abc, where a is the absorbance of the solution, a is the molar. In this equation a is. These factors and assumptions can. Beer's Law In Colorimetry.
From stuff.iorodeo.com
Lab 2 Beer’s Law and Molar Extinction Coefficients — Colorimeter User Beer's Law In Colorimetry Along with operating the instruments, beer's law also involves calculations to actually figure out the concentration of a solution from the. The colorimeter is usually used to measure the concentration of a. It refers to a device which helps specific solutions to absorb a particular wavelength of light. Determining the concentration of a solution. In this equation a is. A. Beer's Law In Colorimetry.
From mungfali.com
UV Spectroscopy Beer Lambert Law Beer's Law In Colorimetry Ensate for the natural color loss of food during storage. In this equation a is. This relationship is known as beer’s law and is given by the equation: Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following. Determining the concentration of a solution. Along with operating the instruments, beer's. Beer's Law In Colorimetry.
From scienceinfo.com
BeerLambert Law Statement, Derivation, Applications, Limitations Beer's Law In Colorimetry This relationship is known as beer’s law and is given by the equation: A = abc, where a is the absorbance of the solution, a is the molar. Beer's law is an equation that relates light's attenuation to a material's properties. The colorimeter is usually used to measure the concentration of a. Along with operating the instruments, beer's law also. Beer's Law In Colorimetry.
From www.youtube.com
Beer Lambert Law Demonstration Tamil Colorimeter Beer's Law In Colorimetry Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following. Ensate for the natural color loss of food during storage. In this equation a is. The colorimeter is usually used to measure the concentration of a. This relationship is known as beer’s law and is given by the equation: A. Beer's Law In Colorimetry.
From www.scienceabc.com
Beers Law Definition, History, Equation, Formula And Example Beer's Law In Colorimetry Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following. Determining the concentration of a solution. A = abc, where a is the absorbance of the solution, a is the molar. These factors and assumptions can be summarized as beer's law and written as the equation, a = abc. Ensate. Beer's Law In Colorimetry.
From www.thoughtco.com
Beer's Law Definition and Equation Beer's Law In Colorimetry This relationship is known as beer’s law and is given by the equation: Ensate for the natural color loss of food during storage. The colorimeter is usually used to measure the concentration of a. In this equation a is. It refers to a device which helps specific solutions to absorb a particular wavelength of light. Beer's law is an equation. Beer's Law In Colorimetry.
From www.slideserve.com
PPT Beer’s Law & Colorimetry PowerPoint Presentation, free download Beer's Law In Colorimetry Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following. In this equation a is. A = abc, where a is the absorbance of the solution, a is the molar. Ensate for the natural color loss of food during storage. This relationship is known as beer’s law and is given. Beer's Law In Colorimetry.
From www.youtube.com
Colorimetry Verify LambertBeer's Law & To find Concentration of Beer's Law In Colorimetry A = abc, where a is the absorbance of the solution, a is the molar. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following. Ensate for the natural color loss of food during storage. In this equation a is. This relationship is known as beer’s law and is given. Beer's Law In Colorimetry.
From slideplayer.com
Colorimetry and Beer’s Law ppt download Beer's Law In Colorimetry A = abc, where a is the absorbance of the solution, a is the molar. Beer's law is an equation that relates light's attenuation to a material's properties. In this equation a is. These factors and assumptions can be summarized as beer's law and written as the equation, a = abc. It refers to a device which helps specific solutions. Beer's Law In Colorimetry.
From rrennenextanner.blogspot.com
Beer Lambert Law Ppt rrennenexTanner Beer's Law In Colorimetry Determining the concentration of a solution. A = abc, where a is the absorbance of the solution, a is the molar. The colorimeter is usually used to measure the concentration of a. Ensate for the natural color loss of food during storage. In this equation a is. These factors and assumptions can be summarized as beer's law and written as. Beer's Law In Colorimetry.
From slideplayer.com
Colorimetry and Beer’s Law ppt download Beer's Law In Colorimetry A = abc, where a is the absorbance of the solution, a is the molar. Beer's law is an equation that relates light's attenuation to a material's properties. Along with operating the instruments, beer's law also involves calculations to actually figure out the concentration of a solution from the. It refers to a device which helps specific solutions to absorb. Beer's Law In Colorimetry.
From www.youtube.com
Spectrophotometry and Beer's Law YouTube Beer's Law In Colorimetry Along with operating the instruments, beer's law also involves calculations to actually figure out the concentration of a solution from the. In this equation a is. Ensate for the natural color loss of food during storage. A = abc, where a is the absorbance of the solution, a is the molar. The colorimeter is usually used to measure the concentration. Beer's Law In Colorimetry.
From www.vernier.com
Determining the Concentration of a Solution Beer's Law > Experiment 17 Beer's Law In Colorimetry The colorimeter is usually used to measure the concentration of a. This relationship is known as beer’s law and is given by the equation: A = abc, where a is the absorbance of the solution, a is the molar. It refers to a device which helps specific solutions to absorb a particular wavelength of light. In this equation a is.. Beer's Law In Colorimetry.
From www.chegg.com
Solved COLORIMETRYBEER'S LAW Transmitancc LAB REPORT 1. Beer's Law In Colorimetry It refers to a device which helps specific solutions to absorb a particular wavelength of light. These factors and assumptions can be summarized as beer's law and written as the equation, a = abc. Ensate for the natural color loss of food during storage. This relationship is known as beer’s law and is given by the equation: The colorimeter is. Beer's Law In Colorimetry.
From www.youtube.com
Spectrophotometer Beer's Law And Colorimetry Lab YouTube Beer's Law In Colorimetry Determining the concentration of a solution. It refers to a device which helps specific solutions to absorb a particular wavelength of light. In this equation a is. Beer's law is an equation that relates light's attenuation to a material's properties. Ensate for the natural color loss of food during storage. The colorimeter is usually used to measure the concentration of. Beer's Law In Colorimetry.
From www.youtube.com
Theory of Colorimetry & Colorimeter Beer's & Lambert's Law YouTube Beer's Law In Colorimetry These factors and assumptions can be summarized as beer's law and written as the equation, a = abc. The colorimeter is usually used to measure the concentration of a. Beer's law is an equation that relates light's attenuation to a material's properties. Ensate for the natural color loss of food during storage. Since the concentration, path length and molar absorptivity. Beer's Law In Colorimetry.
From slideplayer.com
Beer’s Law and Concentrations ppt download Beer's Law In Colorimetry These factors and assumptions can be summarized as beer's law and written as the equation, a = abc. Along with operating the instruments, beer's law also involves calculations to actually figure out the concentration of a solution from the. This relationship is known as beer’s law and is given by the equation: Since the concentration, path length and molar absorptivity. Beer's Law In Colorimetry.
From www.chem.ucla.edu
Beer's Law Tutorial Beer's Law In Colorimetry Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following. It refers to a device which helps specific solutions to absorb a particular wavelength of light. The colorimeter is usually used to measure the concentration of a. Determining the concentration of a solution. Along with operating the instruments, beer's law. Beer's Law In Colorimetry.
From sciencenotes.org
Beer's Law Equation and Example Beer's Law In Colorimetry Ensate for the natural color loss of food during storage. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following. A = abc, where a is the absorbance of the solution, a is the molar. It refers to a device which helps specific solutions to absorb a particular wavelength of. Beer's Law In Colorimetry.
From www.youtube.com
Beer's Law Overview YouTube Beer's Law In Colorimetry A = abc, where a is the absorbance of the solution, a is the molar. These factors and assumptions can be summarized as beer's law and written as the equation, a = abc. It refers to a device which helps specific solutions to absorb a particular wavelength of light. In this equation a is. This relationship is known as beer’s. Beer's Law In Colorimetry.
From slideplayer.com
Beer’s Law Colorimetry Colligative Properties Review ppt download Beer's Law In Colorimetry It refers to a device which helps specific solutions to absorb a particular wavelength of light. Beer's law is an equation that relates light's attenuation to a material's properties. This relationship is known as beer’s law and is given by the equation: Determining the concentration of a solution. A = abc, where a is the absorbance of the solution, a. Beer's Law In Colorimetry.
From www.youtube.com
Working of Colorimeter and Verification of Beer'sLambert's law Beer's Law In Colorimetry Ensate for the natural color loss of food during storage. In this equation a is. These factors and assumptions can be summarized as beer's law and written as the equation, a = abc. Determining the concentration of a solution. This relationship is known as beer’s law and is given by the equation: Beer's law is an equation that relates light's. Beer's Law In Colorimetry.
From www.chegg.com
Solved BEERS LAWTransmi COLORIMETRYBEER'S LAW LAB REPORT Beer's Law In Colorimetry Along with operating the instruments, beer's law also involves calculations to actually figure out the concentration of a solution from the. This relationship is known as beer’s law and is given by the equation: The colorimeter is usually used to measure the concentration of a. These factors and assumptions can be summarized as beer's law and written as the equation,. Beer's Law In Colorimetry.
From slideplayer.com
Beer’s Law Colorimetry Colligative Properties Review ppt download Beer's Law In Colorimetry This relationship is known as beer’s law and is given by the equation: Determining the concentration of a solution. These factors and assumptions can be summarized as beer's law and written as the equation, a = abc. A = abc, where a is the absorbance of the solution, a is the molar. Since the concentration, path length and molar absorptivity. Beer's Law In Colorimetry.
From www.slideshare.net
beer lamberts law, colorimetery, nephlometry and turbidimetry Beer's Law In Colorimetry This relationship is known as beer’s law and is given by the equation: Ensate for the natural color loss of food during storage. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following. It refers to a device which helps specific solutions to absorb a particular wavelength of light. In. Beer's Law In Colorimetry.