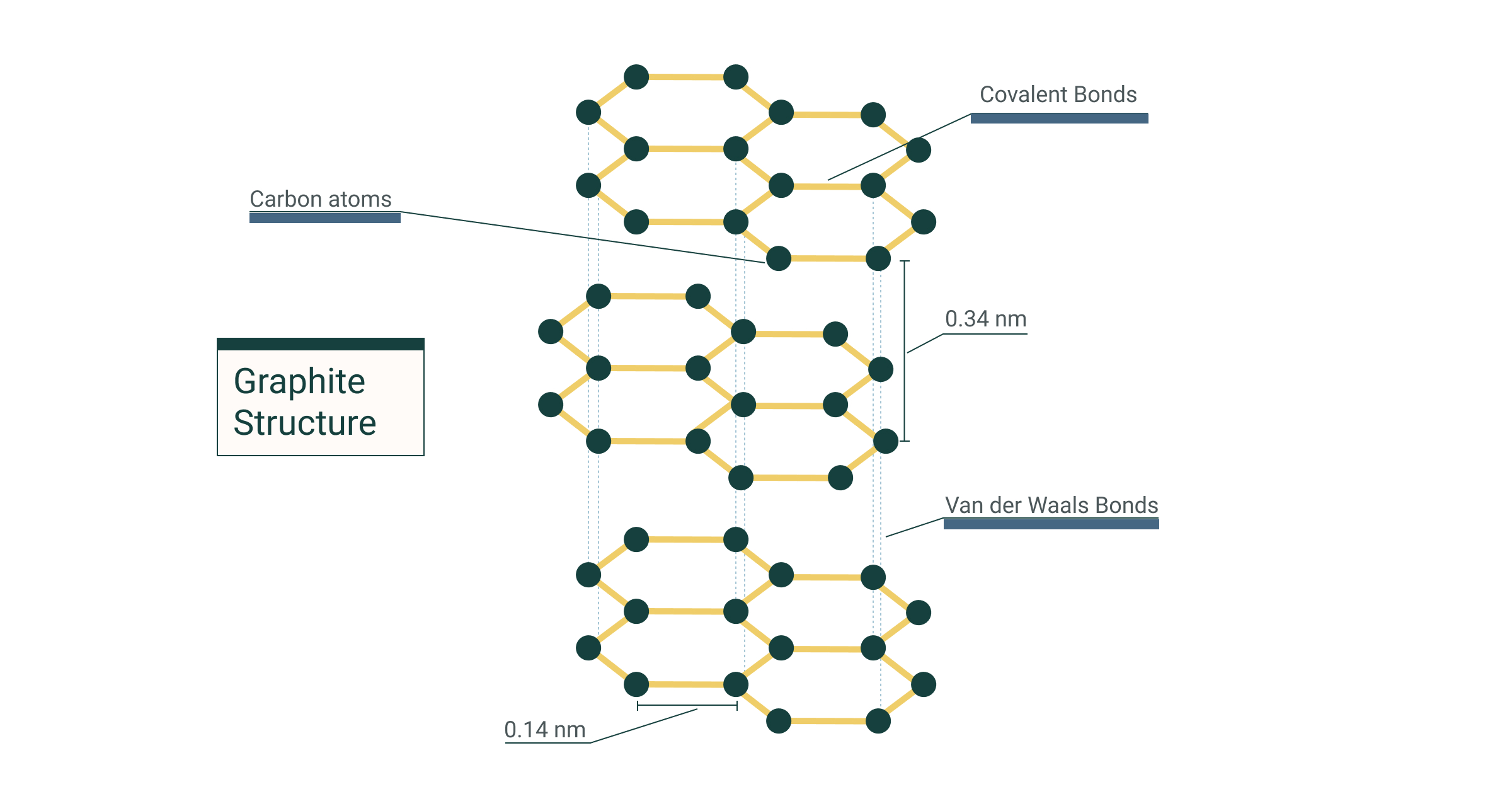
What Is A Giant Lattice Structure . , such as sodium chloride, are arranged in a giant ionic structure (also known. Metals form giant metallic lattices in which the metal ions are surrounded by a ‘sea’ of delocalised electrons. The lattice consists of a regulararrangement of alternating positive and. What is a giant ionic structure? Metals form giant metallic lattices in which the metal ions are surrounded by a ‘sea’ of delocalised electrons. Is a giant structure of ions. Ionic compounds have a giant lattice structure. A giant ionic lattice structure ionic bonds are very strong so the melting point of an ionic compound is high. The ions have a regular, repeating arrangement called an.
from www.breakingatom.com
Metals form giant metallic lattices in which the metal ions are surrounded by a ‘sea’ of delocalised electrons. Ionic compounds have a giant lattice structure. The lattice consists of a regulararrangement of alternating positive and. , such as sodium chloride, are arranged in a giant ionic structure (also known. A giant ionic lattice structure ionic bonds are very strong so the melting point of an ionic compound is high. What is a giant ionic structure? The ions have a regular, repeating arrangement called an. Metals form giant metallic lattices in which the metal ions are surrounded by a ‘sea’ of delocalised electrons. Is a giant structure of ions.
Giant Covalent Compounds
What Is A Giant Lattice Structure A giant ionic lattice structure ionic bonds are very strong so the melting point of an ionic compound is high. What is a giant ionic structure? The lattice consists of a regulararrangement of alternating positive and. Ionic compounds have a giant lattice structure. Is a giant structure of ions. A giant ionic lattice structure ionic bonds are very strong so the melting point of an ionic compound is high. Metals form giant metallic lattices in which the metal ions are surrounded by a ‘sea’ of delocalised electrons. The ions have a regular, repeating arrangement called an. Metals form giant metallic lattices in which the metal ions are surrounded by a ‘sea’ of delocalised electrons. , such as sodium chloride, are arranged in a giant ionic structure (also known.
From www.linstitute.net
Edexcel A Level Chemistry复习笔记1.5.6 Giant Lattices翰林国际教育 What Is A Giant Lattice Structure Is a giant structure of ions. , such as sodium chloride, are arranged in a giant ionic structure (also known. Ionic compounds have a giant lattice structure. The lattice consists of a regulararrangement of alternating positive and. What is a giant ionic structure? A giant ionic lattice structure ionic bonds are very strong so the melting point of an ionic. What Is A Giant Lattice Structure.
From mammothmemory.net
Examples of substances that have giant covalent bonds What Is A Giant Lattice Structure A giant ionic lattice structure ionic bonds are very strong so the melting point of an ionic compound is high. Metals form giant metallic lattices in which the metal ions are surrounded by a ‘sea’ of delocalised electrons. Ionic compounds have a giant lattice structure. The ions have a regular, repeating arrangement called an. Is a giant structure of ions.. What Is A Giant Lattice Structure.
From www.breakingatom.com
Giant Covalent Compounds What Is A Giant Lattice Structure Ionic compounds have a giant lattice structure. The lattice consists of a regulararrangement of alternating positive and. Metals form giant metallic lattices in which the metal ions are surrounded by a ‘sea’ of delocalised electrons. The ions have a regular, repeating arrangement called an. , such as sodium chloride, are arranged in a giant ionic structure (also known. Metals form. What Is A Giant Lattice Structure.
From www.slideserve.com
PPT KS4 Chemistry PowerPoint Presentation, free download ID9350982 What Is A Giant Lattice Structure Is a giant structure of ions. The ions have a regular, repeating arrangement called an. , such as sodium chloride, are arranged in a giant ionic structure (also known. Metals form giant metallic lattices in which the metal ions are surrounded by a ‘sea’ of delocalised electrons. The lattice consists of a regulararrangement of alternating positive and. Metals form giant. What Is A Giant Lattice Structure.
From igcse-chemistry-2017.blogspot.com
IGCSE Chemistry 2017 1.49 Explain Why Substances with Giant Covalent What Is A Giant Lattice Structure , such as sodium chloride, are arranged in a giant ionic structure (also known. What is a giant ionic structure? A giant ionic lattice structure ionic bonds are very strong so the melting point of an ionic compound is high. Metals form giant metallic lattices in which the metal ions are surrounded by a ‘sea’ of delocalised electrons. The lattice. What Is A Giant Lattice Structure.
From www.tec-science.com
Influence of the lattice structure on ductility tecscience What Is A Giant Lattice Structure The lattice consists of a regulararrangement of alternating positive and. The ions have a regular, repeating arrangement called an. Metals form giant metallic lattices in which the metal ions are surrounded by a ‘sea’ of delocalised electrons. Metals form giant metallic lattices in which the metal ions are surrounded by a ‘sea’ of delocalised electrons. , such as sodium chloride,. What Is A Giant Lattice Structure.
From www.researchgate.net
Various architectures of lattice structures (A) Strutbased lattice What Is A Giant Lattice Structure Ionic compounds have a giant lattice structure. Is a giant structure of ions. Metals form giant metallic lattices in which the metal ions are surrounded by a ‘sea’ of delocalised electrons. The ions have a regular, repeating arrangement called an. What is a giant ionic structure? , such as sodium chloride, are arranged in a giant ionic structure (also known.. What Is A Giant Lattice Structure.
From slidetodoc.com
GIANT IONIC LATTICES Ionic Bonding the net electrostatic What Is A Giant Lattice Structure A giant ionic lattice structure ionic bonds are very strong so the melting point of an ionic compound is high. What is a giant ionic structure? Ionic compounds have a giant lattice structure. The ions have a regular, repeating arrangement called an. Is a giant structure of ions. Metals form giant metallic lattices in which the metal ions are surrounded. What Is A Giant Lattice Structure.
From www.hanlin.com
CIE A Level Chemistry复习笔记1.4.3 Lattice Structures翰林国际教育 What Is A Giant Lattice Structure Ionic compounds have a giant lattice structure. Metals form giant metallic lattices in which the metal ions are surrounded by a ‘sea’ of delocalised electrons. Is a giant structure of ions. The lattice consists of a regulararrangement of alternating positive and. Metals form giant metallic lattices in which the metal ions are surrounded by a ‘sea’ of delocalised electrons. What. What Is A Giant Lattice Structure.
From mammothmemory.net
Examples of substances that have giant covalent bonds What Is A Giant Lattice Structure Ionic compounds have a giant lattice structure. The lattice consists of a regulararrangement of alternating positive and. Metals form giant metallic lattices in which the metal ions are surrounded by a ‘sea’ of delocalised electrons. The ions have a regular, repeating arrangement called an. Is a giant structure of ions. , such as sodium chloride, are arranged in a giant. What Is A Giant Lattice Structure.
From www.slideshare.net
Chem matters ch6_ionic_bond What Is A Giant Lattice Structure Is a giant structure of ions. A giant ionic lattice structure ionic bonds are very strong so the melting point of an ionic compound is high. What is a giant ionic structure? Metals form giant metallic lattices in which the metal ions are surrounded by a ‘sea’ of delocalised electrons. Metals form giant metallic lattices in which the metal ions. What Is A Giant Lattice Structure.
From www.tes.com
Giant Ionic Structure GCSE Teaching Resources What Is A Giant Lattice Structure Is a giant structure of ions. The lattice consists of a regulararrangement of alternating positive and. The ions have a regular, repeating arrangement called an. Ionic compounds have a giant lattice structure. What is a giant ionic structure? Metals form giant metallic lattices in which the metal ions are surrounded by a ‘sea’ of delocalised electrons. , such as sodium. What Is A Giant Lattice Structure.
From www.goldhilleducation.co.uk
IGCSE Chemistry Mychem What Is A Giant Lattice Structure The lattice consists of a regulararrangement of alternating positive and. Ionic compounds have a giant lattice structure. The ions have a regular, repeating arrangement called an. Metals form giant metallic lattices in which the metal ions are surrounded by a ‘sea’ of delocalised electrons. A giant ionic lattice structure ionic bonds are very strong so the melting point of an. What Is A Giant Lattice Structure.
From www.chemistrystudent.com
Lattice Enthalpies (Alevel) ChemistryStudent What Is A Giant Lattice Structure Metals form giant metallic lattices in which the metal ions are surrounded by a ‘sea’ of delocalised electrons. The lattice consists of a regulararrangement of alternating positive and. What is a giant ionic structure? Ionic compounds have a giant lattice structure. , such as sodium chloride, are arranged in a giant ionic structure (also known. Metals form giant metallic lattices. What Is A Giant Lattice Structure.
From keystagewiki.com
Giant Ionic Structure Key Stage Wiki What Is A Giant Lattice Structure Is a giant structure of ions. Metals form giant metallic lattices in which the metal ions are surrounded by a ‘sea’ of delocalised electrons. Ionic compounds have a giant lattice structure. The ions have a regular, repeating arrangement called an. The lattice consists of a regulararrangement of alternating positive and. Metals form giant metallic lattices in which the metal ions. What Is A Giant Lattice Structure.
From www.breakingatom.com
Giant Covalent Compounds What Is A Giant Lattice Structure What is a giant ionic structure? Ionic compounds have a giant lattice structure. The ions have a regular, repeating arrangement called an. , such as sodium chloride, are arranged in a giant ionic structure (also known. The lattice consists of a regulararrangement of alternating positive and. Is a giant structure of ions. Metals form giant metallic lattices in which the. What Is A Giant Lattice Structure.
From brilliant.org
chemical bonding Brilliant Math & Science Wiki What Is A Giant Lattice Structure What is a giant ionic structure? Ionic compounds have a giant lattice structure. The lattice consists of a regulararrangement of alternating positive and. Metals form giant metallic lattices in which the metal ions are surrounded by a ‘sea’ of delocalised electrons. A giant ionic lattice structure ionic bonds are very strong so the melting point of an ionic compound is. What Is A Giant Lattice Structure.
From courses.lumenlearning.com
Lattice Structures in Crystalline Solids Chemistry Atoms First What Is A Giant Lattice Structure Metals form giant metallic lattices in which the metal ions are surrounded by a ‘sea’ of delocalised electrons. , such as sodium chloride, are arranged in a giant ionic structure (also known. Is a giant structure of ions. The ions have a regular, repeating arrangement called an. The lattice consists of a regulararrangement of alternating positive and. A giant ionic. What Is A Giant Lattice Structure.
From www.tec-science.com
Important types of lattice structures tecscience What Is A Giant Lattice Structure , such as sodium chloride, are arranged in a giant ionic structure (also known. A giant ionic lattice structure ionic bonds are very strong so the melting point of an ionic compound is high. The ions have a regular, repeating arrangement called an. Metals form giant metallic lattices in which the metal ions are surrounded by a ‘sea’ of delocalised. What Is A Giant Lattice Structure.
From www.slideserve.com
PPT 1.6 Bonding PowerPoint Presentation, free download ID3692914 What Is A Giant Lattice Structure Is a giant structure of ions. What is a giant ionic structure? The ions have a regular, repeating arrangement called an. , such as sodium chloride, are arranged in a giant ionic structure (also known. Metals form giant metallic lattices in which the metal ions are surrounded by a ‘sea’ of delocalised electrons. Ionic compounds have a giant lattice structure.. What Is A Giant Lattice Structure.
From www.revisechemistry.uk
Bonding and Properties of materials OCR Gateway C2 revisechemistry.uk What Is A Giant Lattice Structure Ionic compounds have a giant lattice structure. Metals form giant metallic lattices in which the metal ions are surrounded by a ‘sea’ of delocalised electrons. Metals form giant metallic lattices in which the metal ions are surrounded by a ‘sea’ of delocalised electrons. What is a giant ionic structure? The lattice consists of a regulararrangement of alternating positive and. The. What Is A Giant Lattice Structure.
From www.slideserve.com
PPT Giant Ionic Structures PowerPoint Presentation, free download What Is A Giant Lattice Structure Is a giant structure of ions. Metals form giant metallic lattices in which the metal ions are surrounded by a ‘sea’ of delocalised electrons. , such as sodium chloride, are arranged in a giant ionic structure (also known. Metals form giant metallic lattices in which the metal ions are surrounded by a ‘sea’ of delocalised electrons. The lattice consists of. What Is A Giant Lattice Structure.
From www.youtube.com
mr i explains Giant Ionic Structures YouTube What Is A Giant Lattice Structure Ionic compounds have a giant lattice structure. What is a giant ionic structure? A giant ionic lattice structure ionic bonds are very strong so the melting point of an ionic compound is high. The lattice consists of a regulararrangement of alternating positive and. Metals form giant metallic lattices in which the metal ions are surrounded by a ‘sea’ of delocalised. What Is A Giant Lattice Structure.
From parametrichouse.com
Lattice Structure Parametric House What Is A Giant Lattice Structure The ions have a regular, repeating arrangement called an. Metals form giant metallic lattices in which the metal ions are surrounded by a ‘sea’ of delocalised electrons. Metals form giant metallic lattices in which the metal ions are surrounded by a ‘sea’ of delocalised electrons. A giant ionic lattice structure ionic bonds are very strong so the melting point of. What Is A Giant Lattice Structure.
From www.youtube.com
Giant Ionic and Giant Covalent Compounds GCSE Science Chemistry What Is A Giant Lattice Structure The ions have a regular, repeating arrangement called an. , such as sodium chloride, are arranged in a giant ionic structure (also known. A giant ionic lattice structure ionic bonds are very strong so the melting point of an ionic compound is high. Metals form giant metallic lattices in which the metal ions are surrounded by a ‘sea’ of delocalised. What Is A Giant Lattice Structure.
From www.vrogue.co
The Crystal Lattice Structure Of Ionic Compounds Info vrogue.co What Is A Giant Lattice Structure Ionic compounds have a giant lattice structure. Metals form giant metallic lattices in which the metal ions are surrounded by a ‘sea’ of delocalised electrons. A giant ionic lattice structure ionic bonds are very strong so the melting point of an ionic compound is high. , such as sodium chloride, are arranged in a giant ionic structure (also known. What. What Is A Giant Lattice Structure.
From wahibo.com
C1.2.6 Giant covalent structures (AQA) Wahibo Education What Is A Giant Lattice Structure , such as sodium chloride, are arranged in a giant ionic structure (also known. Ionic compounds have a giant lattice structure. What is a giant ionic structure? Metals form giant metallic lattices in which the metal ions are surrounded by a ‘sea’ of delocalised electrons. Metals form giant metallic lattices in which the metal ions are surrounded by a ‘sea’. What Is A Giant Lattice Structure.
From www.shutterstock.com
Vektor Stok Crystal Lattice Structure Ionic Compounds Ionic (Tanpa What Is A Giant Lattice Structure Metals form giant metallic lattices in which the metal ions are surrounded by a ‘sea’ of delocalised electrons. Metals form giant metallic lattices in which the metal ions are surrounded by a ‘sea’ of delocalised electrons. Ionic compounds have a giant lattice structure. , such as sodium chloride, are arranged in a giant ionic structure (also known. The ions have. What Is A Giant Lattice Structure.
From igcse-chemistry-2017.blogspot.com
IGCSE Chemistry 2017 1.52C Know How to Represent a Metallic Lattice What Is A Giant Lattice Structure Metals form giant metallic lattices in which the metal ions are surrounded by a ‘sea’ of delocalised electrons. The ions have a regular, repeating arrangement called an. A giant ionic lattice structure ionic bonds are very strong so the melting point of an ionic compound is high. Ionic compounds have a giant lattice structure. What is a giant ionic structure?. What Is A Giant Lattice Structure.
From study.com
Crystal Lattice Definition & Structure Video & Lesson Transcript What Is A Giant Lattice Structure , such as sodium chloride, are arranged in a giant ionic structure (also known. The ions have a regular, repeating arrangement called an. A giant ionic lattice structure ionic bonds are very strong so the melting point of an ionic compound is high. Ionic compounds have a giant lattice structure. The lattice consists of a regulararrangement of alternating positive and.. What Is A Giant Lattice Structure.
From igcse-chemistry-2017.blogspot.com
IGCSE Chemistry 2017 1.42 Understand Why Compounds with Giant Ionic What Is A Giant Lattice Structure Ionic compounds have a giant lattice structure. A giant ionic lattice structure ionic bonds are very strong so the melting point of an ionic compound is high. Is a giant structure of ions. , such as sodium chloride, are arranged in a giant ionic structure (also known. Metals form giant metallic lattices in which the metal ions are surrounded by. What Is A Giant Lattice Structure.
From mmerevise.co.uk
Giant Covalent Structures, Polymers, and Structures of Carbon MME What Is A Giant Lattice Structure A giant ionic lattice structure ionic bonds are very strong so the melting point of an ionic compound is high. Is a giant structure of ions. Ionic compounds have a giant lattice structure. Metals form giant metallic lattices in which the metal ions are surrounded by a ‘sea’ of delocalised electrons. The ions have a regular, repeating arrangement called an.. What Is A Giant Lattice Structure.
From www.studypool.com
SOLUTION Giant Covalent Structures Presentation Studypool What Is A Giant Lattice Structure Ionic compounds have a giant lattice structure. The ions have a regular, repeating arrangement called an. Metals form giant metallic lattices in which the metal ions are surrounded by a ‘sea’ of delocalised electrons. A giant ionic lattice structure ionic bonds are very strong so the melting point of an ionic compound is high. The lattice consists of a regulararrangement. What Is A Giant Lattice Structure.
From www.tec-science.com
Important types of lattice structures tecscience What Is A Giant Lattice Structure Metals form giant metallic lattices in which the metal ions are surrounded by a ‘sea’ of delocalised electrons. , such as sodium chloride, are arranged in a giant ionic structure (also known. The ions have a regular, repeating arrangement called an. A giant ionic lattice structure ionic bonds are very strong so the melting point of an ionic compound is. What Is A Giant Lattice Structure.
From www.thesciencehive.co.uk
Ionic Bonding — the science hive What Is A Giant Lattice Structure , such as sodium chloride, are arranged in a giant ionic structure (also known. A giant ionic lattice structure ionic bonds are very strong so the melting point of an ionic compound is high. Is a giant structure of ions. Metals form giant metallic lattices in which the metal ions are surrounded by a ‘sea’ of delocalised electrons. The ions. What Is A Giant Lattice Structure.