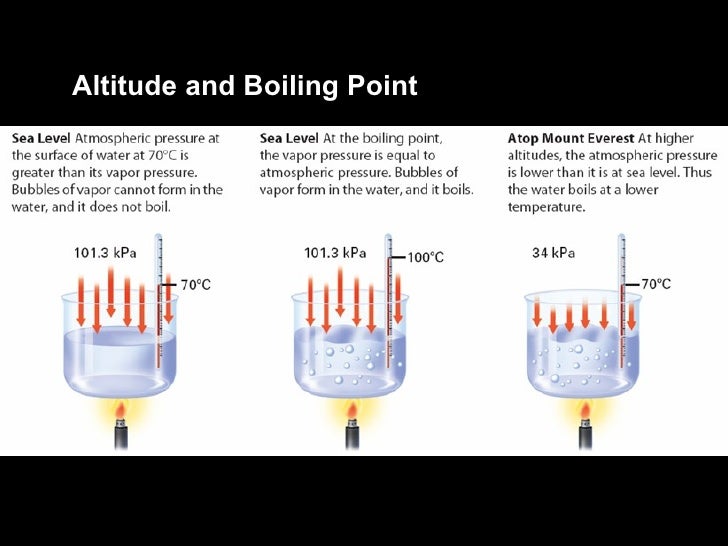
Does Water Boil Faster At Higher Altitude . More energy, as in higher heat, makes molecules move even faster. Less energy means less heat, which means water will boil at a lower temperature at a higher altitude. One definition of boiling point is the temperature at which the substance's vapour pressure (the pressure inside the bubble) equals. At a higher elevation, the lower atmospheric pressure means heated water reaches its boiling point more quickly—i.e., at a lower temperature. At high altitudes, water boils at lower temperatures, so you need to boil it for longer to ensure it’s safe to drink: Water at sea level boils at 212 degrees fahrenheit;. When atmospheric pressure is lower, such as at a higher altitude, it takes less energy to bring water to the boiling point. The thing is, if you boil water in high. More specifically, it affects a very important component of cooking: When you boil water, you're literally speeding up liquid h20 molecules so much that you're breaking their bonds and letting.
from www.slideshare.net
More specifically, it affects a very important component of cooking: The thing is, if you boil water in high. One definition of boiling point is the temperature at which the substance's vapour pressure (the pressure inside the bubble) equals. Less energy means less heat, which means water will boil at a lower temperature at a higher altitude. At a higher elevation, the lower atmospheric pressure means heated water reaches its boiling point more quickly—i.e., at a lower temperature. Water at sea level boils at 212 degrees fahrenheit;. At high altitudes, water boils at lower temperatures, so you need to boil it for longer to ensure it’s safe to drink: When you boil water, you're literally speeding up liquid h20 molecules so much that you're breaking their bonds and letting. When atmospheric pressure is lower, such as at a higher altitude, it takes less energy to bring water to the boiling point. More energy, as in higher heat, makes molecules move even faster.
Lecture 13.2 Liquids
Does Water Boil Faster At Higher Altitude More specifically, it affects a very important component of cooking: At high altitudes, water boils at lower temperatures, so you need to boil it for longer to ensure it’s safe to drink: When you boil water, you're literally speeding up liquid h20 molecules so much that you're breaking their bonds and letting. Water at sea level boils at 212 degrees fahrenheit;. At a higher elevation, the lower atmospheric pressure means heated water reaches its boiling point more quickly—i.e., at a lower temperature. More specifically, it affects a very important component of cooking: When atmospheric pressure is lower, such as at a higher altitude, it takes less energy to bring water to the boiling point. One definition of boiling point is the temperature at which the substance's vapour pressure (the pressure inside the bubble) equals. The thing is, if you boil water in high. Less energy means less heat, which means water will boil at a lower temperature at a higher altitude. More energy, as in higher heat, makes molecules move even faster.
From www.tec-science.com
Why does water boil faster at high altitudes? tecscience Does Water Boil Faster At Higher Altitude More specifically, it affects a very important component of cooking: At high altitudes, water boils at lower temperatures, so you need to boil it for longer to ensure it’s safe to drink: At a higher elevation, the lower atmospheric pressure means heated water reaches its boiling point more quickly—i.e., at a lower temperature. The thing is, if you boil water. Does Water Boil Faster At Higher Altitude.
From www.tec-science.com
Why does water boil faster at high altitudes? tecscience Does Water Boil Faster At Higher Altitude At a higher elevation, the lower atmospheric pressure means heated water reaches its boiling point more quickly—i.e., at a lower temperature. When you boil water, you're literally speeding up liquid h20 molecules so much that you're breaking their bonds and letting. When atmospheric pressure is lower, such as at a higher altitude, it takes less energy to bring water to. Does Water Boil Faster At Higher Altitude.
From surfguppy.com
How does Atmospheric Pressure Affect Boiling Point Does Water Boil Faster At Higher Altitude When atmospheric pressure is lower, such as at a higher altitude, it takes less energy to bring water to the boiling point. The thing is, if you boil water in high. One definition of boiling point is the temperature at which the substance's vapour pressure (the pressure inside the bubble) equals. Less energy means less heat, which means water will. Does Water Boil Faster At Higher Altitude.
From www.tastingtable.com
How To Adjust Your Water Boiling Process For High Altitudes Does Water Boil Faster At Higher Altitude More specifically, it affects a very important component of cooking: When atmospheric pressure is lower, such as at a higher altitude, it takes less energy to bring water to the boiling point. The thing is, if you boil water in high. Less energy means less heat, which means water will boil at a lower temperature at a higher altitude. Water. Does Water Boil Faster At Higher Altitude.
From www.tec-science.com
Why does water boil faster at high altitudes? tecscience Does Water Boil Faster At Higher Altitude When you boil water, you're literally speeding up liquid h20 molecules so much that you're breaking their bonds and letting. Water at sea level boils at 212 degrees fahrenheit;. At high altitudes, water boils at lower temperatures, so you need to boil it for longer to ensure it’s safe to drink: More energy, as in higher heat, makes molecules move. Does Water Boil Faster At Higher Altitude.
From brainly.in
Q9. Why does water boil at a lower temperature at higher altitudes Does Water Boil Faster At Higher Altitude More specifically, it affects a very important component of cooking: The thing is, if you boil water in high. At high altitudes, water boils at lower temperatures, so you need to boil it for longer to ensure it’s safe to drink: When atmospheric pressure is lower, such as at a higher altitude, it takes less energy to bring water to. Does Water Boil Faster At Higher Altitude.
From exotahhqc.blob.core.windows.net
Water Boils Faster At High Altitudes at Rosa Smith blog Does Water Boil Faster At Higher Altitude More energy, as in higher heat, makes molecules move even faster. The thing is, if you boil water in high. One definition of boiling point is the temperature at which the substance's vapour pressure (the pressure inside the bubble) equals. When atmospheric pressure is lower, such as at a higher altitude, it takes less energy to bring water to the. Does Water Boil Faster At Higher Altitude.
From medium.com
Does water freeze or boil in space? Starts With A Bang! Medium Does Water Boil Faster At Higher Altitude At high altitudes, water boils at lower temperatures, so you need to boil it for longer to ensure it’s safe to drink: At a higher elevation, the lower atmospheric pressure means heated water reaches its boiling point more quickly—i.e., at a lower temperature. Water at sea level boils at 212 degrees fahrenheit;. One definition of boiling point is the temperature. Does Water Boil Faster At Higher Altitude.
From www.youtube.com
Why does water boils faster at higher altitudes ? In English YouTube Does Water Boil Faster At Higher Altitude At high altitudes, water boils at lower temperatures, so you need to boil it for longer to ensure it’s safe to drink: When you boil water, you're literally speeding up liquid h20 molecules so much that you're breaking their bonds and letting. More specifically, it affects a very important component of cooking: When atmospheric pressure is lower, such as at. Does Water Boil Faster At Higher Altitude.
From loetufefz.blob.core.windows.net
How Can Water Boil Faster at Saul Liu blog Does Water Boil Faster At Higher Altitude When you boil water, you're literally speeding up liquid h20 molecules so much that you're breaking their bonds and letting. At high altitudes, water boils at lower temperatures, so you need to boil it for longer to ensure it’s safe to drink: Less energy means less heat, which means water will boil at a lower temperature at a higher altitude.. Does Water Boil Faster At Higher Altitude.
From exotahhqc.blob.core.windows.net
Water Boils Faster At High Altitudes at Rosa Smith blog Does Water Boil Faster At Higher Altitude At high altitudes, water boils at lower temperatures, so you need to boil it for longer to ensure it’s safe to drink: More specifically, it affects a very important component of cooking: When atmospheric pressure is lower, such as at a higher altitude, it takes less energy to bring water to the boiling point. At a higher elevation, the lower. Does Water Boil Faster At Higher Altitude.
From www.scienceabc.com
Why Does Water Boil Quickly At High Altitudes? » ScienceABC Does Water Boil Faster At Higher Altitude At high altitudes, water boils at lower temperatures, so you need to boil it for longer to ensure it’s safe to drink: When you boil water, you're literally speeding up liquid h20 molecules so much that you're breaking their bonds and letting. At a higher elevation, the lower atmospheric pressure means heated water reaches its boiling point more quickly—i.e., at. Does Water Boil Faster At Higher Altitude.
From www.cuisinecravings.com
Does Water Boil Faster With a Lid? How to Boil Water Faster? Does Water Boil Faster At Higher Altitude The thing is, if you boil water in high. Water at sea level boils at 212 degrees fahrenheit;. One definition of boiling point is the temperature at which the substance's vapour pressure (the pressure inside the bubble) equals. When atmospheric pressure is lower, such as at a higher altitude, it takes less energy to bring water to the boiling point.. Does Water Boil Faster At Higher Altitude.
From www.myopencountry.com
Boiling Water at Higher Altitude What You Need to Know My Open Country Does Water Boil Faster At Higher Altitude At a higher elevation, the lower atmospheric pressure means heated water reaches its boiling point more quickly—i.e., at a lower temperature. More specifically, it affects a very important component of cooking: The thing is, if you boil water in high. When you boil water, you're literally speeding up liquid h20 molecules so much that you're breaking their bonds and letting.. Does Water Boil Faster At Higher Altitude.
From dxolovigi.blob.core.windows.net
Why Does Water Boil At A Lower Temperature In A Vacuum at Marcy Miller blog Does Water Boil Faster At Higher Altitude At high altitudes, water boils at lower temperatures, so you need to boil it for longer to ensure it’s safe to drink: At a higher elevation, the lower atmospheric pressure means heated water reaches its boiling point more quickly—i.e., at a lower temperature. One definition of boiling point is the temperature at which the substance's vapour pressure (the pressure inside. Does Water Boil Faster At Higher Altitude.
From www.wonderopolis.org
Why Does Water Boil Faster at Higher Altitude? Wonderopolis Does Water Boil Faster At Higher Altitude When atmospheric pressure is lower, such as at a higher altitude, it takes less energy to bring water to the boiling point. Water at sea level boils at 212 degrees fahrenheit;. When you boil water, you're literally speeding up liquid h20 molecules so much that you're breaking their bonds and letting. More energy, as in higher heat, makes molecules move. Does Water Boil Faster At Higher Altitude.
From chefcamper.com
Why does water boil at lower temperatures at higher altitudes? Does Water Boil Faster At Higher Altitude One definition of boiling point is the temperature at which the substance's vapour pressure (the pressure inside the bubble) equals. At a higher elevation, the lower atmospheric pressure means heated water reaches its boiling point more quickly—i.e., at a lower temperature. The thing is, if you boil water in high. More specifically, it affects a very important component of cooking:. Does Water Boil Faster At Higher Altitude.
From joibgircm.blob.core.windows.net
Temperature To Boil Water At Altitude at Richard Centeno blog Does Water Boil Faster At Higher Altitude More energy, as in higher heat, makes molecules move even faster. One definition of boiling point is the temperature at which the substance's vapour pressure (the pressure inside the bubble) equals. At a higher elevation, the lower atmospheric pressure means heated water reaches its boiling point more quickly—i.e., at a lower temperature. At high altitudes, water boils at lower temperatures,. Does Water Boil Faster At Higher Altitude.
From aweseas.blogspot.com
Boiling Point Of Water Sea Level Does Water Boil Faster At Higher Altitude At a higher elevation, the lower atmospheric pressure means heated water reaches its boiling point more quickly—i.e., at a lower temperature. At high altitudes, water boils at lower temperatures, so you need to boil it for longer to ensure it’s safe to drink: One definition of boiling point is the temperature at which the substance's vapour pressure (the pressure inside. Does Water Boil Faster At Higher Altitude.
From wonderopolis.org
Why Does Water Boil Faster at Higher Altitude? Wonderopolis Does Water Boil Faster At Higher Altitude When you boil water, you're literally speeding up liquid h20 molecules so much that you're breaking their bonds and letting. At a higher elevation, the lower atmospheric pressure means heated water reaches its boiling point more quickly—i.e., at a lower temperature. Water at sea level boils at 212 degrees fahrenheit;. When atmospheric pressure is lower, such as at a higher. Does Water Boil Faster At Higher Altitude.
From www.kindpng.com
Peppa Pig Banned Does Water Boil Faster At Higher Altitudes, HD Png Does Water Boil Faster At Higher Altitude One definition of boiling point is the temperature at which the substance's vapour pressure (the pressure inside the bubble) equals. Less energy means less heat, which means water will boil at a lower temperature at a higher altitude. When you boil water, you're literally speeding up liquid h20 molecules so much that you're breaking their bonds and letting. More energy,. Does Water Boil Faster At Higher Altitude.
From www.thespruceeats.com
The Boiling Point of Water at Various Altitudes Does Water Boil Faster At Higher Altitude When you boil water, you're literally speeding up liquid h20 molecules so much that you're breaking their bonds and letting. When atmospheric pressure is lower, such as at a higher altitude, it takes less energy to bring water to the boiling point. More energy, as in higher heat, makes molecules move even faster. One definition of boiling point is the. Does Water Boil Faster At Higher Altitude.
From www.pinterest.com
Why Does Water Boil Faster at Higher Altitude? The voice, Your voice Does Water Boil Faster At Higher Altitude At high altitudes, water boils at lower temperatures, so you need to boil it for longer to ensure it’s safe to drink: More energy, as in higher heat, makes molecules move even faster. One definition of boiling point is the temperature at which the substance's vapour pressure (the pressure inside the bubble) equals. Water at sea level boils at 212. Does Water Boil Faster At Higher Altitude.
From www.slideshare.net
Lecture 13.2 Liquids Does Water Boil Faster At Higher Altitude More energy, as in higher heat, makes molecules move even faster. Water at sea level boils at 212 degrees fahrenheit;. More specifically, it affects a very important component of cooking: The thing is, if you boil water in high. At high altitudes, water boils at lower temperatures, so you need to boil it for longer to ensure it’s safe to. Does Water Boil Faster At Higher Altitude.
From joimizjtn.blob.core.windows.net
Which Boils Faster Saltwater Or Regular Water at Mary Currier blog Does Water Boil Faster At Higher Altitude The thing is, if you boil water in high. When you boil water, you're literally speeding up liquid h20 molecules so much that you're breaking their bonds and letting. At a higher elevation, the lower atmospheric pressure means heated water reaches its boiling point more quickly—i.e., at a lower temperature. When atmospheric pressure is lower, such as at a higher. Does Water Boil Faster At Higher Altitude.
From www.tec-science.com
Why does water boil faster at high altitudes? tecscience Does Water Boil Faster At Higher Altitude The thing is, if you boil water in high. At a higher elevation, the lower atmospheric pressure means heated water reaches its boiling point more quickly—i.e., at a lower temperature. Less energy means less heat, which means water will boil at a lower temperature at a higher altitude. One definition of boiling point is the temperature at which the substance's. Does Water Boil Faster At Higher Altitude.
From www.wave3.com
Behind the Forecast Outwitting the weather when baking Does Water Boil Faster At Higher Altitude When atmospheric pressure is lower, such as at a higher altitude, it takes less energy to bring water to the boiling point. More energy, as in higher heat, makes molecules move even faster. One definition of boiling point is the temperature at which the substance's vapour pressure (the pressure inside the bubble) equals. When you boil water, you're literally speeding. Does Water Boil Faster At Higher Altitude.
From exotahhqc.blob.core.windows.net
Water Boils Faster At High Altitudes at Rosa Smith blog Does Water Boil Faster At Higher Altitude When you boil water, you're literally speeding up liquid h20 molecules so much that you're breaking their bonds and letting. One definition of boiling point is the temperature at which the substance's vapour pressure (the pressure inside the bubble) equals. Water at sea level boils at 212 degrees fahrenheit;. At high altitudes, water boils at lower temperatures, so you need. Does Water Boil Faster At Higher Altitude.
From www.scienceabc.com
Why Does Water Boil Quickly At High Altitudes? » ScienceABC Does Water Boil Faster At Higher Altitude More specifically, it affects a very important component of cooking: Less energy means less heat, which means water will boil at a lower temperature at a higher altitude. One definition of boiling point is the temperature at which the substance's vapour pressure (the pressure inside the bubble) equals. At high altitudes, water boils at lower temperatures, so you need to. Does Water Boil Faster At Higher Altitude.
From www.compoundchem.com
What Temperature Does Water Boil At? Boiling Point & Elevation Does Water Boil Faster At Higher Altitude At a higher elevation, the lower atmospheric pressure means heated water reaches its boiling point more quickly—i.e., at a lower temperature. More specifically, it affects a very important component of cooking: Water at sea level boils at 212 degrees fahrenheit;. Less energy means less heat, which means water will boil at a lower temperature at a higher altitude. The thing. Does Water Boil Faster At Higher Altitude.
From joibgircm.blob.core.windows.net
Temperature To Boil Water At Altitude at Richard Centeno blog Does Water Boil Faster At Higher Altitude Less energy means less heat, which means water will boil at a lower temperature at a higher altitude. The thing is, if you boil water in high. When atmospheric pressure is lower, such as at a higher altitude, it takes less energy to bring water to the boiling point. At a higher elevation, the lower atmospheric pressure means heated water. Does Water Boil Faster At Higher Altitude.
From www.learnatnoon.com
The boiling point of water and alcohol explained Noon Academy Does Water Boil Faster At Higher Altitude When you boil water, you're literally speeding up liquid h20 molecules so much that you're breaking their bonds and letting. More energy, as in higher heat, makes molecules move even faster. Water at sea level boils at 212 degrees fahrenheit;. At a higher elevation, the lower atmospheric pressure means heated water reaches its boiling point more quickly—i.e., at a lower. Does Water Boil Faster At Higher Altitude.
From www.tec-science.com
Why does water boil faster at high altitudes? tecscience Does Water Boil Faster At Higher Altitude More energy, as in higher heat, makes molecules move even faster. One definition of boiling point is the temperature at which the substance's vapour pressure (the pressure inside the bubble) equals. When you boil water, you're literally speeding up liquid h20 molecules so much that you're breaking their bonds and letting. The thing is, if you boil water in high.. Does Water Boil Faster At Higher Altitude.
From exotahhqc.blob.core.windows.net
Water Boils Faster At High Altitudes at Rosa Smith blog Does Water Boil Faster At Higher Altitude The thing is, if you boil water in high. More specifically, it affects a very important component of cooking: At a higher elevation, the lower atmospheric pressure means heated water reaches its boiling point more quickly—i.e., at a lower temperature. One definition of boiling point is the temperature at which the substance's vapour pressure (the pressure inside the bubble) equals.. Does Water Boil Faster At Higher Altitude.
From slideplayer.com
Chapter 3 Review. ppt download Does Water Boil Faster At Higher Altitude At a higher elevation, the lower atmospheric pressure means heated water reaches its boiling point more quickly—i.e., at a lower temperature. One definition of boiling point is the temperature at which the substance's vapour pressure (the pressure inside the bubble) equals. Water at sea level boils at 212 degrees fahrenheit;. At high altitudes, water boils at lower temperatures, so you. Does Water Boil Faster At Higher Altitude.