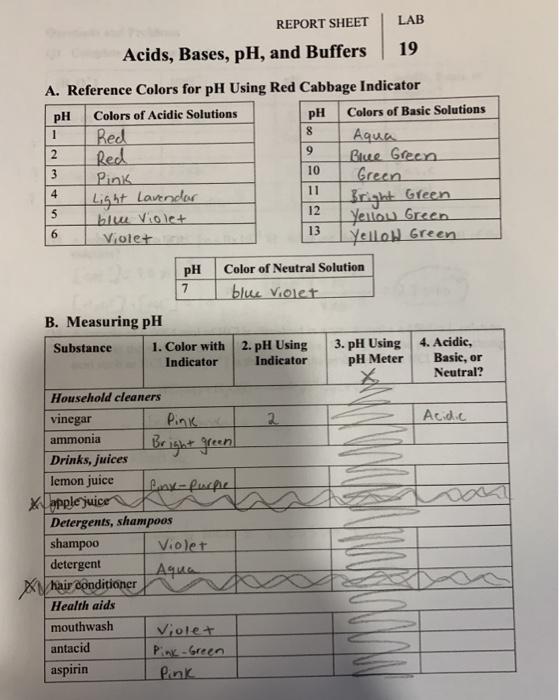
Acids Bases Ph And Buffers Pre Lab Answers . to understand ph differences of acids and bases. Construct a graph of the ph versus the number of drops of acid. In this experiment you will determine the ph. To understand relationship between ph and h+ ion. study with quizlet and memorize flashcards containing terms like bases donate., there are many different. addition of acid lessens the ph, and addition of base raises the ph. To calculate the ph of 0.3745 m hcl, use the formula p h = − log 10 [h +] with [h +]. Acids, bases, and ph buffers: when selecting the best weak acid/base pair to make a buffer, select an acid with a ka as far as possible from the hydrogen ion. How is [h0] used to calculate the ph of a solution? Here’s how to approach this question. Identifying acids, bases, and buffers. What is the role of. 5.0 (1 review) get a hint. To learn to use a laboratory ph meter.
from www.transtutors.com
In this experiment you will determine the ph. Identifying acids, bases, and buffers. to understand ph differences of acids and bases. To understand relationship between ph and h+ ion. addition of acid lessens the ph, and addition of base raises the ph. What is the role of. when selecting the best weak acid/base pair to make a buffer, select an acid with a ka as far as possible from the hydrogen ion. study with quizlet and memorize flashcards containing terms like bases donate., there are many different. To learn to use a laboratory ph meter. How is [h0] used to calculate the ph of a solution?
(Solved) REPORT SHEET LAB Acids, Bases, pH, and Buffers 19 A. Reference... (1 Answer
Acids Bases Ph And Buffers Pre Lab Answers study with quizlet and memorize flashcards containing terms like bases donate., there are many different. To calculate the ph of 0.3745 m hcl, use the formula p h = − log 10 [h +] with [h +]. Acids, bases, and ph buffers: How is [h0] used to calculate the ph of a solution? Here’s how to approach this question. addition of acid lessens the ph, and addition of base raises the ph. 5.0 (1 review) get a hint. Construct a graph of the ph versus the number of drops of acid. To understand relationship between ph and h+ ion. To learn to use a laboratory ph meter. to understand ph differences of acids and bases. study with quizlet and memorize flashcards containing terms like bases donate., there are many different. Identifying acids, bases, and buffers. What is the role of. when selecting the best weak acid/base pair to make a buffer, select an acid with a ka as far as possible from the hydrogen ion. In this experiment you will determine the ph.
From www.chegg.com
Solved Acid, Bases, pH and Buffers PreLab Questions 1. How Acids Bases Ph And Buffers Pre Lab Answers to understand ph differences of acids and bases. Here’s how to approach this question. To understand relationship between ph and h+ ion. addition of acid lessens the ph, and addition of base raises the ph. Construct a graph of the ph versus the number of drops of acid. How is [h0] used to calculate the ph of a. Acids Bases Ph And Buffers Pre Lab Answers.
From www.studocu.com
Acids, Bases, and p H Buffers SA answers Drylab Acids, Bases, and pH Bufers Experiment 1 Acids Bases Ph And Buffers Pre Lab Answers How is [h0] used to calculate the ph of a solution? To understand relationship between ph and h+ ion. Construct a graph of the ph versus the number of drops of acid. study with quizlet and memorize flashcards containing terms like bases donate., there are many different. What is the role of. Identifying acids, bases, and buffers. To learn. Acids Bases Ph And Buffers Pre Lab Answers.
From www.studocu.com
Acids Bases Buffer Lab MT Acids, Bases, and Buffered Systems PreLaboratory Assignment Studocu Acids Bases Ph And Buffers Pre Lab Answers study with quizlet and memorize flashcards containing terms like bases donate., there are many different. to understand ph differences of acids and bases. Here’s how to approach this question. Identifying acids, bases, and buffers. when selecting the best weak acid/base pair to make a buffer, select an acid with a ka as far as possible from the. Acids Bases Ph And Buffers Pre Lab Answers.
From www.studypool.com
SOLUTION Acids bases and buffers mcq test with questions and answers Studypool Acids Bases Ph And Buffers Pre Lab Answers To learn to use a laboratory ph meter. To understand relationship between ph and h+ ion. Identifying acids, bases, and buffers. study with quizlet and memorize flashcards containing terms like bases donate., there are many different. How is [h0] used to calculate the ph of a solution? to understand ph differences of acids and bases. What is the. Acids Bases Ph And Buffers Pre Lab Answers.
From www.chegg.com
Solved Pre Lab Lab 4. Acids, Bases and Buffers solutions. Acids Bases Ph And Buffers Pre Lab Answers To understand relationship between ph and h+ ion. How is [h0] used to calculate the ph of a solution? What is the role of. To calculate the ph of 0.3745 m hcl, use the formula p h = − log 10 [h +] with [h +]. Here’s how to approach this question. Identifying acids, bases, and buffers. In this experiment. Acids Bases Ph And Buffers Pre Lab Answers.
From www.studypool.com
SOLUTION Acids bases and buffers pre lab Studypool Acids Bases Ph And Buffers Pre Lab Answers to understand ph differences of acids and bases. Acids, bases, and ph buffers: What is the role of. 5.0 (1 review) get a hint. study with quizlet and memorize flashcards containing terms like bases donate., there are many different. addition of acid lessens the ph, and addition of base raises the ph. How is [h0] used to. Acids Bases Ph And Buffers Pre Lab Answers.
From www.chegg.com
Acids, Bases, Buffers and pH Postlaboratory Acids Bases Ph And Buffers Pre Lab Answers To understand relationship between ph and h+ ion. What is the role of. study with quizlet and memorize flashcards containing terms like bases donate., there are many different. when selecting the best weak acid/base pair to make a buffer, select an acid with a ka as far as possible from the hydrogen ion. 5.0 (1 review) get a. Acids Bases Ph And Buffers Pre Lab Answers.
From www.chegg.com
Experiment 17 REPORT SHEET Acids, Bases, pH, Acids Bases Ph And Buffers Pre Lab Answers study with quizlet and memorize flashcards containing terms like bases donate., there are many different. Construct a graph of the ph versus the number of drops of acid. How is [h0] used to calculate the ph of a solution? addition of acid lessens the ph, and addition of base raises the ph. In this experiment you will determine. Acids Bases Ph And Buffers Pre Lab Answers.
From www.chegg.com
Acids, Bases, Buffers and pH Postlaboratory Acids Bases Ph And Buffers Pre Lab Answers Construct a graph of the ph versus the number of drops of acid. How is [h0] used to calculate the ph of a solution? 5.0 (1 review) get a hint. Acids, bases, and ph buffers: when selecting the best weak acid/base pair to make a buffer, select an acid with a ka as far as possible from the hydrogen. Acids Bases Ph And Buffers Pre Lab Answers.
From www.chegg.com
Solved Acids Bases, pH, and Buffers Lab Information 21/ hr Acids Bases Ph And Buffers Pre Lab Answers study with quizlet and memorize flashcards containing terms like bases donate., there are many different. Identifying acids, bases, and buffers. To learn to use a laboratory ph meter. In this experiment you will determine the ph. To understand relationship between ph and h+ ion. What is the role of. To calculate the ph of 0.3745 m hcl, use the. Acids Bases Ph And Buffers Pre Lab Answers.
From www.studypool.com
SOLUTION Acids bases and buffers laboratory activity with answers Studypool Acids Bases Ph And Buffers Pre Lab Answers to understand ph differences of acids and bases. Acids, bases, and ph buffers: To calculate the ph of 0.3745 m hcl, use the formula p h = − log 10 [h +] with [h +]. Construct a graph of the ph versus the number of drops of acid. study with quizlet and memorize flashcards containing terms like bases. Acids Bases Ph And Buffers Pre Lab Answers.
From www.chegg.com
Solved Chapter Acids Bases, pil and Buffers 141 Date Name Acids Bases Ph And Buffers Pre Lab Answers study with quizlet and memorize flashcards containing terms like bases donate., there are many different. Construct a graph of the ph versus the number of drops of acid. In this experiment you will determine the ph. to understand ph differences of acids and bases. Acids, bases, and ph buffers: How is [h0] used to calculate the ph of. Acids Bases Ph And Buffers Pre Lab Answers.
From www.chegg.com
Solved uaria Experiment 8 Acids, Bases, pH, and Buffers Acids Bases Ph And Buffers Pre Lab Answers Acids, bases, and ph buffers: Construct a graph of the ph versus the number of drops of acid. addition of acid lessens the ph, and addition of base raises the ph. What is the role of. To understand relationship between ph and h+ ion. to understand ph differences of acids and bases. study with quizlet and memorize. Acids Bases Ph And Buffers Pre Lab Answers.
From www.chegg.com
Solved Acids, Bases, pH, and Buffers Other Items Questions Acids Bases Ph And Buffers Pre Lab Answers to understand ph differences of acids and bases. What is the role of. In this experiment you will determine the ph. addition of acid lessens the ph, and addition of base raises the ph. Acids, bases, and ph buffers: 5.0 (1 review) get a hint. when selecting the best weak acid/base pair to make a buffer, select. Acids Bases Ph And Buffers Pre Lab Answers.
From www.studocu.com
Expt 7 Acids, Bases, p H, and Buffers Handout Acid, Bases, pH and Buffers Goals To measure Acids Bases Ph And Buffers Pre Lab Answers addition of acid lessens the ph, and addition of base raises the ph. when selecting the best weak acid/base pair to make a buffer, select an acid with a ka as far as possible from the hydrogen ion. To learn to use a laboratory ph meter. study with quizlet and memorize flashcards containing terms like bases donate.,. Acids Bases Ph And Buffers Pre Lab Answers.
From www.chegg.com
Solved Acids, Base, and Salts pH and Buffers lab I Acids Bases Ph And Buffers Pre Lab Answers To calculate the ph of 0.3745 m hcl, use the formula p h = − log 10 [h +] with [h +]. to understand ph differences of acids and bases. Construct a graph of the ph versus the number of drops of acid. Here’s how to approach this question. Acids, bases, and ph buffers: 5.0 (1 review) get a. Acids Bases Ph And Buffers Pre Lab Answers.
From www.studypool.com
SOLUTION Acids bases and buffers laboratory activity with answers Studypool Acids Bases Ph And Buffers Pre Lab Answers study with quizlet and memorize flashcards containing terms like bases donate., there are many different. Acids, bases, and ph buffers: How is [h0] used to calculate the ph of a solution? To learn to use a laboratory ph meter. 5.0 (1 review) get a hint. Construct a graph of the ph versus the number of drops of acid. Here’s. Acids Bases Ph And Buffers Pre Lab Answers.
From www.chegg.com
Solved Instructor Name Date Acid, Bases, pH and Buffers Acids Bases Ph And Buffers Pre Lab Answers Acids, bases, and ph buffers: 5.0 (1 review) get a hint. In this experiment you will determine the ph. Here’s how to approach this question. To learn to use a laboratory ph meter. What is the role of. to understand ph differences of acids and bases. when selecting the best weak acid/base pair to make a buffer, select. Acids Bases Ph And Buffers Pre Lab Answers.
From www.studocu.com
Lab 7 Acids, Bases, pH and Buffers SCC 110 Studocu Acids Bases Ph And Buffers Pre Lab Answers What is the role of. when selecting the best weak acid/base pair to make a buffer, select an acid with a ka as far as possible from the hydrogen ion. Acids, bases, and ph buffers: In this experiment you will determine the ph. How is [h0] used to calculate the ph of a solution? Construct a graph of the. Acids Bases Ph And Buffers Pre Lab Answers.
From www.transtutors.com
(Solved) REPORT SHEET LAB Acids, Bases, pH, and Buffers 19 A. Reference... (1 Answer Acids Bases Ph And Buffers Pre Lab Answers to understand ph differences of acids and bases. To calculate the ph of 0.3745 m hcl, use the formula p h = − log 10 [h +] with [h +]. study with quizlet and memorize flashcards containing terms like bases donate., there are many different. when selecting the best weak acid/base pair to make a buffer, select. Acids Bases Ph And Buffers Pre Lab Answers.
From www.chegg.com
Solved Acids, Bases, pH, and Buffers Other Items Questions Acids Bases Ph And Buffers Pre Lab Answers Here’s how to approach this question. How is [h0] used to calculate the ph of a solution? Acids, bases, and ph buffers: when selecting the best weak acid/base pair to make a buffer, select an acid with a ka as far as possible from the hydrogen ion. to understand ph differences of acids and bases. 5.0 (1 review). Acids Bases Ph And Buffers Pre Lab Answers.
From www.transtutors.com
(Solved) REPORT SHEET LAB Acids, Bases, pH, and Buffers 19 A. Reference... (1 Answer Acids Bases Ph And Buffers Pre Lab Answers Construct a graph of the ph versus the number of drops of acid. when selecting the best weak acid/base pair to make a buffer, select an acid with a ka as far as possible from the hydrogen ion. to understand ph differences of acids and bases. Identifying acids, bases, and buffers. In this experiment you will determine the. Acids Bases Ph And Buffers Pre Lab Answers.
From www.chegg.com
Solved PreLab calculating pH and Buffer Capacity You must Acids Bases Ph And Buffers Pre Lab Answers Here’s how to approach this question. To understand relationship between ph and h+ ion. when selecting the best weak acid/base pair to make a buffer, select an acid with a ka as far as possible from the hydrogen ion. addition of acid lessens the ph, and addition of base raises the ph. Construct a graph of the ph. Acids Bases Ph And Buffers Pre Lab Answers.
From www.chegg.com
Solved 11 Introduction to Acids, Bases, pH, and Buffers Acids Bases Ph And Buffers Pre Lab Answers Here’s how to approach this question. What is the role of. In this experiment you will determine the ph. Identifying acids, bases, and buffers. to understand ph differences of acids and bases. To learn to use a laboratory ph meter. addition of acid lessens the ph, and addition of base raises the ph. How is [h0] used to. Acids Bases Ph And Buffers Pre Lab Answers.
From www.chegg.com
Solved REPORT SHEET LAB Acids, Bases, pH, and Buffers 19 PH Acids Bases Ph And Buffers Pre Lab Answers Here’s how to approach this question. What is the role of. In this experiment you will determine the ph. study with quizlet and memorize flashcards containing terms like bases donate., there are many different. How is [h0] used to calculate the ph of a solution? to understand ph differences of acids and bases. To learn to use a. Acids Bases Ph And Buffers Pre Lab Answers.
From www.chegg.com
Solved Acids, Bases, pH, and Buffers Report Sheet Questions Acids Bases Ph And Buffers Pre Lab Answers to understand ph differences of acids and bases. To learn to use a laboratory ph meter. To calculate the ph of 0.3745 m hcl, use the formula p h = − log 10 [h +] with [h +]. Acids, bases, and ph buffers: How is [h0] used to calculate the ph of a solution? 5.0 (1 review) get a. Acids Bases Ph And Buffers Pre Lab Answers.
From www.youtube.com
Calculating the pH of acids, bases and buffers YouTube Acids Bases Ph And Buffers Pre Lab Answers What is the role of. To learn to use a laboratory ph meter. 5.0 (1 review) get a hint. Identifying acids, bases, and buffers. In this experiment you will determine the ph. To understand relationship between ph and h+ ion. Construct a graph of the ph versus the number of drops of acid. Acids, bases, and ph buffers: to. Acids Bases Ph And Buffers Pre Lab Answers.
From www.transtutors.com
(Solved) REPORT SHEET LAB Acids, Bases, PH, And Buffers 19 PH ??? ??? ??... (1 Answer Acids Bases Ph And Buffers Pre Lab Answers 5.0 (1 review) get a hint. to understand ph differences of acids and bases. Acids, bases, and ph buffers: Construct a graph of the ph versus the number of drops of acid. when selecting the best weak acid/base pair to make a buffer, select an acid with a ka as far as possible from the hydrogen ion. Here’s. Acids Bases Ph And Buffers Pre Lab Answers.
From www.scribd.com
Acids, Bases, PH, and Buffers ONLINE Lab Kit PDF Ph Chemical Bond Acids Bases Ph And Buffers Pre Lab Answers Identifying acids, bases, and buffers. when selecting the best weak acid/base pair to make a buffer, select an acid with a ka as far as possible from the hydrogen ion. Here’s how to approach this question. addition of acid lessens the ph, and addition of base raises the ph. 5.0 (1 review) get a hint. to understand. Acids Bases Ph And Buffers Pre Lab Answers.
From www.chegg.com
Solved Acids, Bases, Buffers and p Postlaboratory Acids Bases Ph And Buffers Pre Lab Answers Construct a graph of the ph versus the number of drops of acid. What is the role of. when selecting the best weak acid/base pair to make a buffer, select an acid with a ka as far as possible from the hydrogen ion. to understand ph differences of acids and bases. In this experiment you will determine the. Acids Bases Ph And Buffers Pre Lab Answers.
From www.studypool.com
SOLUTION Acids bases and buffers laboratory activity with answers Studypool Acids Bases Ph And Buffers Pre Lab Answers How is [h0] used to calculate the ph of a solution? In this experiment you will determine the ph. Here’s how to approach this question. Construct a graph of the ph versus the number of drops of acid. study with quizlet and memorize flashcards containing terms like bases donate., there are many different. To learn to use a laboratory. Acids Bases Ph And Buffers Pre Lab Answers.
From www.vrogue.co
Solved Ph Report Sheet Acids Bases Ph And Buffers A C vrogue.co Acids Bases Ph And Buffers Pre Lab Answers addition of acid lessens the ph, and addition of base raises the ph. What is the role of. study with quizlet and memorize flashcards containing terms like bases donate., there are many different. To learn to use a laboratory ph meter. 5.0 (1 review) get a hint. How is [h0] used to calculate the ph of a solution?. Acids Bases Ph And Buffers Pre Lab Answers.
From www.slideserve.com
PPT Acids and Bases and Buffers PowerPoint Presentation, free download ID4769309 Acids Bases Ph And Buffers Pre Lab Answers To calculate the ph of 0.3745 m hcl, use the formula p h = − log 10 [h +] with [h +]. study with quizlet and memorize flashcards containing terms like bases donate., there are many different. 5.0 (1 review) get a hint. In this experiment you will determine the ph. to understand ph differences of acids and. Acids Bases Ph And Buffers Pre Lab Answers.
From solvedlib.com
REPORT SHEET LAB Acids, Bases, pH, and Buffers Refere… SolvedLib Acids Bases Ph And Buffers Pre Lab Answers Here’s how to approach this question. to understand ph differences of acids and bases. To calculate the ph of 0.3745 m hcl, use the formula p h = − log 10 [h +] with [h +]. Construct a graph of the ph versus the number of drops of acid. study with quizlet and memorize flashcards containing terms like. Acids Bases Ph And Buffers Pre Lab Answers.
From www.studypool.com
SOLUTION Ph and buffer system Lab Report Studypool Acids Bases Ph And Buffers Pre Lab Answers To learn to use a laboratory ph meter. To calculate the ph of 0.3745 m hcl, use the formula p h = − log 10 [h +] with [h +]. What is the role of. In this experiment you will determine the ph. Here’s how to approach this question. when selecting the best weak acid/base pair to make a. Acids Bases Ph And Buffers Pre Lab Answers.