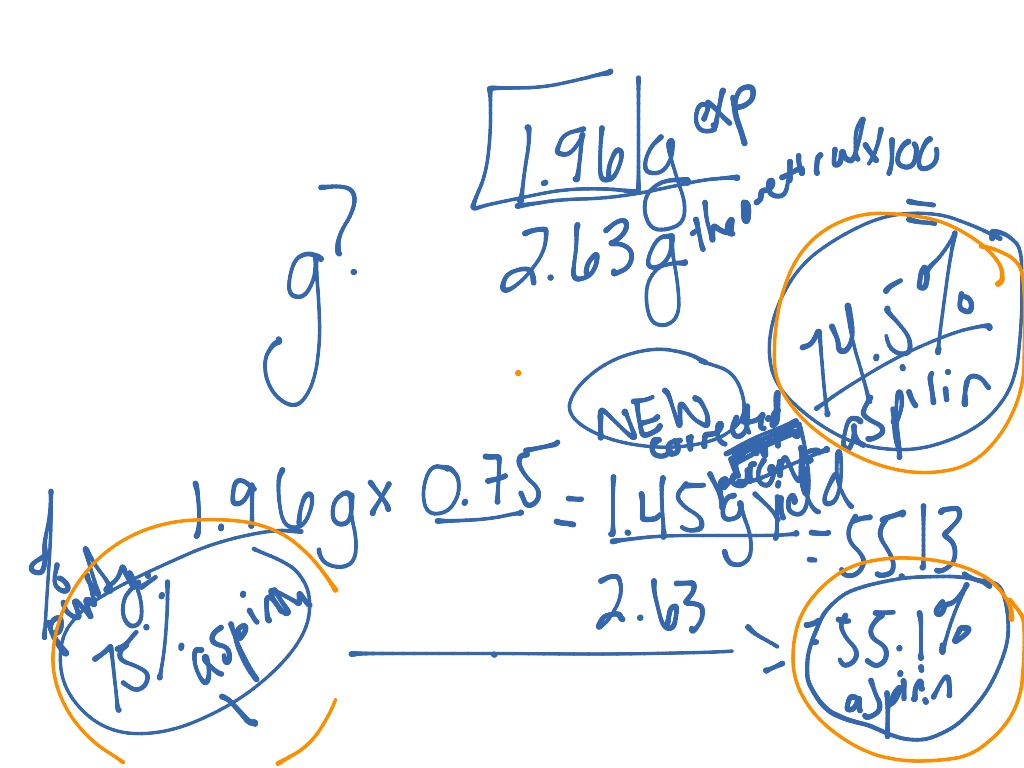
Aspirin Percent Calculation . In this experiment you will measure the amount of aspirin produced and calculate the percent yield. This action is not available. You calculate the theoretical yield of aspirin, and then you use your actual yield to calculate the percent yield. Add 5 ml (0.05 mole) of acetic anhydride, followed by 5 drops of conc. In this experiment you will calculate the limiting reagent and the percent yield for the reaction in the synthesis of aspirin. Forecast the potential impact of different interventions on. Chemical reactions will continue as long as there. When calculating the yield of product, the calculation must always be based on the limiting reagent, not the reagents that are in excess.
from www.showme.com
In this experiment you will measure the amount of aspirin produced and calculate the percent yield. In this experiment you will calculate the limiting reagent and the percent yield for the reaction in the synthesis of aspirin. Chemical reactions will continue as long as there. Forecast the potential impact of different interventions on. You calculate the theoretical yield of aspirin, and then you use your actual yield to calculate the percent yield. Add 5 ml (0.05 mole) of acetic anhydride, followed by 5 drops of conc. This action is not available. When calculating the yield of product, the calculation must always be based on the limiting reagent, not the reagents that are in excess.
Aspirin Percent Yield vs Percent Purity Calculations Science
Aspirin Percent Calculation Add 5 ml (0.05 mole) of acetic anhydride, followed by 5 drops of conc. Add 5 ml (0.05 mole) of acetic anhydride, followed by 5 drops of conc. This action is not available. When calculating the yield of product, the calculation must always be based on the limiting reagent, not the reagents that are in excess. Forecast the potential impact of different interventions on. In this experiment you will measure the amount of aspirin produced and calculate the percent yield. In this experiment you will calculate the limiting reagent and the percent yield for the reaction in the synthesis of aspirin. You calculate the theoretical yield of aspirin, and then you use your actual yield to calculate the percent yield. Chemical reactions will continue as long as there.
From www.numerade.com
SOLVED Calculate the theoretical yield of aspirin (FW = 180.158 g/mol Aspirin Percent Calculation You calculate the theoretical yield of aspirin, and then you use your actual yield to calculate the percent yield. Chemical reactions will continue as long as there. This action is not available. In this experiment you will calculate the limiting reagent and the percent yield for the reaction in the synthesis of aspirin. Add 5 ml (0.05 mole) of acetic. Aspirin Percent Calculation.
From www.chegg.com
Solved Calculate the percent purity of aspirin in each Aspirin Percent Calculation Add 5 ml (0.05 mole) of acetic anhydride, followed by 5 drops of conc. This action is not available. Forecast the potential impact of different interventions on. You calculate the theoretical yield of aspirin, and then you use your actual yield to calculate the percent yield. In this experiment you will calculate the limiting reagent and the percent yield for. Aspirin Percent Calculation.
From www.youtube.com
Aspirin Percent Yield Math YouTube Aspirin Percent Calculation You calculate the theoretical yield of aspirin, and then you use your actual yield to calculate the percent yield. Add 5 ml (0.05 mole) of acetic anhydride, followed by 5 drops of conc. This action is not available. Chemical reactions will continue as long as there. In this experiment you will measure the amount of aspirin produced and calculate the. Aspirin Percent Calculation.
From www.coursehero.com
[Solved] Data Sheet for Aspirin Synthesis Laboratory Calculate the Aspirin Percent Calculation When calculating the yield of product, the calculation must always be based on the limiting reagent, not the reagents that are in excess. Chemical reactions will continue as long as there. Forecast the potential impact of different interventions on. In this experiment you will calculate the limiting reagent and the percent yield for the reaction in the synthesis of aspirin.. Aspirin Percent Calculation.
From www.toppr.com
Calculate the mass percentage of aspirin (C9H8O4) in acetonitrile Aspirin Percent Calculation In this experiment you will calculate the limiting reagent and the percent yield for the reaction in the synthesis of aspirin. Chemical reactions will continue as long as there. When calculating the yield of product, the calculation must always be based on the limiting reagent, not the reagents that are in excess. In this experiment you will measure the amount. Aspirin Percent Calculation.
From www.chegg.com
Solved Calculate the percent purity of aspirin in each Aspirin Percent Calculation This action is not available. Chemical reactions will continue as long as there. You calculate the theoretical yield of aspirin, and then you use your actual yield to calculate the percent yield. In this experiment you will measure the amount of aspirin produced and calculate the percent yield. Forecast the potential impact of different interventions on. When calculating the yield. Aspirin Percent Calculation.
From webapi.bu.edu
Calculate the percent yield of aspirin. How do you calculate the Aspirin Percent Calculation Add 5 ml (0.05 mole) of acetic anhydride, followed by 5 drops of conc. In this experiment you will calculate the limiting reagent and the percent yield for the reaction in the synthesis of aspirin. You calculate the theoretical yield of aspirin, and then you use your actual yield to calculate the percent yield. In this experiment you will measure. Aspirin Percent Calculation.
From www.studypool.com
SOLUTION Calculate the percent yield for the above reaction if the Aspirin Percent Calculation In this experiment you will measure the amount of aspirin produced and calculate the percent yield. Forecast the potential impact of different interventions on. In this experiment you will calculate the limiting reagent and the percent yield for the reaction in the synthesis of aspirin. You calculate the theoretical yield of aspirin, and then you use your actual yield to. Aspirin Percent Calculation.
From www.chegg.com
Solved 4. Calculate theoretical yield of aspirin if you Aspirin Percent Calculation This action is not available. In this experiment you will measure the amount of aspirin produced and calculate the percent yield. Chemical reactions will continue as long as there. Add 5 ml (0.05 mole) of acetic anhydride, followed by 5 drops of conc. When calculating the yield of product, the calculation must always be based on the limiting reagent, not. Aspirin Percent Calculation.
From www.slideserve.com
PPT Unit 37 Stoichiometry of formula PowerPoint Presentation, free Aspirin Percent Calculation In this experiment you will calculate the limiting reagent and the percent yield for the reaction in the synthesis of aspirin. You calculate the theoretical yield of aspirin, and then you use your actual yield to calculate the percent yield. This action is not available. When calculating the yield of product, the calculation must always be based on the limiting. Aspirin Percent Calculation.
From www.chegg.com
Solved 17. Calculate the percent composition of aspirin, Aspirin Percent Calculation You calculate the theoretical yield of aspirin, and then you use your actual yield to calculate the percent yield. Chemical reactions will continue as long as there. Add 5 ml (0.05 mole) of acetic anhydride, followed by 5 drops of conc. In this experiment you will measure the amount of aspirin produced and calculate the percent yield. When calculating the. Aspirin Percent Calculation.
From www.doubtnut.com
Calculate the mass percentage of aspirin (C(9)H(8)O(4)) in acetonitril Aspirin Percent Calculation You calculate the theoretical yield of aspirin, and then you use your actual yield to calculate the percent yield. In this experiment you will measure the amount of aspirin produced and calculate the percent yield. In this experiment you will calculate the limiting reagent and the percent yield for the reaction in the synthesis of aspirin. Add 5 ml (0.05. Aspirin Percent Calculation.
From www.coursehero.com
[Solved] Calculate the theoretical yield of aspirin if 3.687 g of Aspirin Percent Calculation Forecast the potential impact of different interventions on. Add 5 ml (0.05 mole) of acetic anhydride, followed by 5 drops of conc. When calculating the yield of product, the calculation must always be based on the limiting reagent, not the reagents that are in excess. In this experiment you will measure the amount of aspirin produced and calculate the percent. Aspirin Percent Calculation.
From www.toppr.com
Calculate the mass percentage of aspirin (C9H8O4) in acetonitrile Aspirin Percent Calculation In this experiment you will measure the amount of aspirin produced and calculate the percent yield. Chemical reactions will continue as long as there. Add 5 ml (0.05 mole) of acetic anhydride, followed by 5 drops of conc. Forecast the potential impact of different interventions on. You calculate the theoretical yield of aspirin, and then you use your actual yield. Aspirin Percent Calculation.
From www.youtube.com
Learn how to calculate the average mass of aspirin in tablets using a Aspirin Percent Calculation Add 5 ml (0.05 mole) of acetic anhydride, followed by 5 drops of conc. In this experiment you will calculate the limiting reagent and the percent yield for the reaction in the synthesis of aspirin. Forecast the potential impact of different interventions on. When calculating the yield of product, the calculation must always be based on the limiting reagent, not. Aspirin Percent Calculation.
From www.chegg.com
Solved Is it the right way to calculate for aspirin Aspirin Percent Calculation When calculating the yield of product, the calculation must always be based on the limiting reagent, not the reagents that are in excess. Add 5 ml (0.05 mole) of acetic anhydride, followed by 5 drops of conc. This action is not available. Forecast the potential impact of different interventions on. You calculate the theoretical yield of aspirin, and then you. Aspirin Percent Calculation.
From www.numerade.com
SOLVED An aspirn sample Was synthesized and analyzed The data for the Aspirin Percent Calculation Forecast the potential impact of different interventions on. You calculate the theoretical yield of aspirin, and then you use your actual yield to calculate the percent yield. This action is not available. When calculating the yield of product, the calculation must always be based on the limiting reagent, not the reagents that are in excess. In this experiment you will. Aspirin Percent Calculation.
From www.youtube.com
theoretical yield of aspirin YouTube Aspirin Percent Calculation Forecast the potential impact of different interventions on. In this experiment you will calculate the limiting reagent and the percent yield for the reaction in the synthesis of aspirin. This action is not available. Chemical reactions will continue as long as there. When calculating the yield of product, the calculation must always be based on the limiting reagent, not the. Aspirin Percent Calculation.
From www.coursehero.com
[Solved] how should I calculate the percentage purity of aspirin Aspirin Percent Calculation When calculating the yield of product, the calculation must always be based on the limiting reagent, not the reagents that are in excess. This action is not available. In this experiment you will measure the amount of aspirin produced and calculate the percent yield. You calculate the theoretical yield of aspirin, and then you use your actual yield to calculate. Aspirin Percent Calculation.
From www.coursehero.com
[Solved] Calculate your percent yield of acetylsalicylic acid. Does Aspirin Percent Calculation You calculate the theoretical yield of aspirin, and then you use your actual yield to calculate the percent yield. Chemical reactions will continue as long as there. This action is not available. In this experiment you will measure the amount of aspirin produced and calculate the percent yield. When calculating the yield of product, the calculation must always be based. Aspirin Percent Calculation.
From www.numerade.com
(a) Calculate the percent ionization of a 0.20 M solution of the Aspirin Percent Calculation Add 5 ml (0.05 mole) of acetic anhydride, followed by 5 drops of conc. In this experiment you will measure the amount of aspirin produced and calculate the percent yield. Forecast the potential impact of different interventions on. This action is not available. Chemical reactions will continue as long as there. When calculating the yield of product, the calculation must. Aspirin Percent Calculation.
From askfilo.com
Q.1.(i) Calculate the mass percentage of aspirin (C9 H8 O4 ) in acetonit.. Aspirin Percent Calculation Chemical reactions will continue as long as there. In this experiment you will measure the amount of aspirin produced and calculate the percent yield. This action is not available. Forecast the potential impact of different interventions on. You calculate the theoretical yield of aspirin, and then you use your actual yield to calculate the percent yield. When calculating the yield. Aspirin Percent Calculation.
From www.numerade.com
SOLVED ' Need help!! In this experiment, salicylic acid is the Aspirin Percent Calculation In this experiment you will calculate the limiting reagent and the percent yield for the reaction in the synthesis of aspirin. When calculating the yield of product, the calculation must always be based on the limiting reagent, not the reagents that are in excess. Add 5 ml (0.05 mole) of acetic anhydride, followed by 5 drops of conc. This action. Aspirin Percent Calculation.
From www.chegg.com
Solved How do you solve for the theoretical yield of Aspirin Percent Calculation When calculating the yield of product, the calculation must always be based on the limiting reagent, not the reagents that are in excess. Chemical reactions will continue as long as there. You calculate the theoretical yield of aspirin, and then you use your actual yield to calculate the percent yield. In this experiment you will measure the amount of aspirin. Aspirin Percent Calculation.
From cityraven.com
😀 How to calculate the theoretical yield of aspirin. How To Calculate Aspirin Percent Calculation When calculating the yield of product, the calculation must always be based on the limiting reagent, not the reagents that are in excess. You calculate the theoretical yield of aspirin, and then you use your actual yield to calculate the percent yield. This action is not available. Forecast the potential impact of different interventions on. Add 5 ml (0.05 mole). Aspirin Percent Calculation.
From www.chegg.com
Solved 1. Calculation of the Percent Yield of Aspirin a) Aspirin Percent Calculation In this experiment you will measure the amount of aspirin produced and calculate the percent yield. You calculate the theoretical yield of aspirin, and then you use your actual yield to calculate the percent yield. When calculating the yield of product, the calculation must always be based on the limiting reagent, not the reagents that are in excess. Add 5. Aspirin Percent Calculation.
From www.numerade.com
SOLVED 4[ 4 * 5 DO Calculate the mass of the pure acetylsalicylic acid Aspirin Percent Calculation When calculating the yield of product, the calculation must always be based on the limiting reagent, not the reagents that are in excess. In this experiment you will measure the amount of aspirin produced and calculate the percent yield. Chemical reactions will continue as long as there. This action is not available. Add 5 ml (0.05 mole) of acetic anhydride,. Aspirin Percent Calculation.
From www.researchgate.net
Standard curve of aspirin for calculation of amount of drug in the Aspirin Percent Calculation In this experiment you will measure the amount of aspirin produced and calculate the percent yield. You calculate the theoretical yield of aspirin, and then you use your actual yield to calculate the percent yield. Add 5 ml (0.05 mole) of acetic anhydride, followed by 5 drops of conc. When calculating the yield of product, the calculation must always be. Aspirin Percent Calculation.
From www.coursehero.com
[Solved] Data Sheet for Aspirin Synthesis Laboratory Calculate the Aspirin Percent Calculation Forecast the potential impact of different interventions on. In this experiment you will measure the amount of aspirin produced and calculate the percent yield. Add 5 ml (0.05 mole) of acetic anhydride, followed by 5 drops of conc. When calculating the yield of product, the calculation must always be based on the limiting reagent, not the reagents that are in. Aspirin Percent Calculation.
From www.coursehero.com
[Solved] how should I calculate the percentage purity of aspirin Aspirin Percent Calculation When calculating the yield of product, the calculation must always be based on the limiting reagent, not the reagents that are in excess. You calculate the theoretical yield of aspirin, and then you use your actual yield to calculate the percent yield. Forecast the potential impact of different interventions on. This action is not available. Chemical reactions will continue as. Aspirin Percent Calculation.
From www.coursehero.com
[Solved] Data and Results Preparation of Aspirin Calculate the Aspirin Percent Calculation In this experiment you will calculate the limiting reagent and the percent yield for the reaction in the synthesis of aspirin. Chemical reactions will continue as long as there. When calculating the yield of product, the calculation must always be based on the limiting reagent, not the reagents that are in excess. This action is not available. You calculate the. Aspirin Percent Calculation.
From webapi.bu.edu
Calculate the percent yield of aspirin. How do you calculate the Aspirin Percent Calculation Forecast the potential impact of different interventions on. You calculate the theoretical yield of aspirin, and then you use your actual yield to calculate the percent yield. Add 5 ml (0.05 mole) of acetic anhydride, followed by 5 drops of conc. This action is not available. In this experiment you will measure the amount of aspirin produced and calculate the. Aspirin Percent Calculation.
From www.youtube.com
How to Calculate the Molar Mass of C9H8O4 Aspirin (Acetylsalicylic Aspirin Percent Calculation You calculate the theoretical yield of aspirin, and then you use your actual yield to calculate the percent yield. In this experiment you will measure the amount of aspirin produced and calculate the percent yield. Chemical reactions will continue as long as there. In this experiment you will calculate the limiting reagent and the percent yield for the reaction in. Aspirin Percent Calculation.
From www.coursehero.com
[Solved] Calculate the percent yield of Aspirin prepared from 2.45 Aspirin Percent Calculation In this experiment you will measure the amount of aspirin produced and calculate the percent yield. In this experiment you will calculate the limiting reagent and the percent yield for the reaction in the synthesis of aspirin. Forecast the potential impact of different interventions on. When calculating the yield of product, the calculation must always be based on the limiting. Aspirin Percent Calculation.
From www.showme.com
Aspirin Percent Yield vs Percent Purity Calculations Science Aspirin Percent Calculation Forecast the potential impact of different interventions on. You calculate the theoretical yield of aspirin, and then you use your actual yield to calculate the percent yield. In this experiment you will calculate the limiting reagent and the percent yield for the reaction in the synthesis of aspirin. Add 5 ml (0.05 mole) of acetic anhydride, followed by 5 drops. Aspirin Percent Calculation.