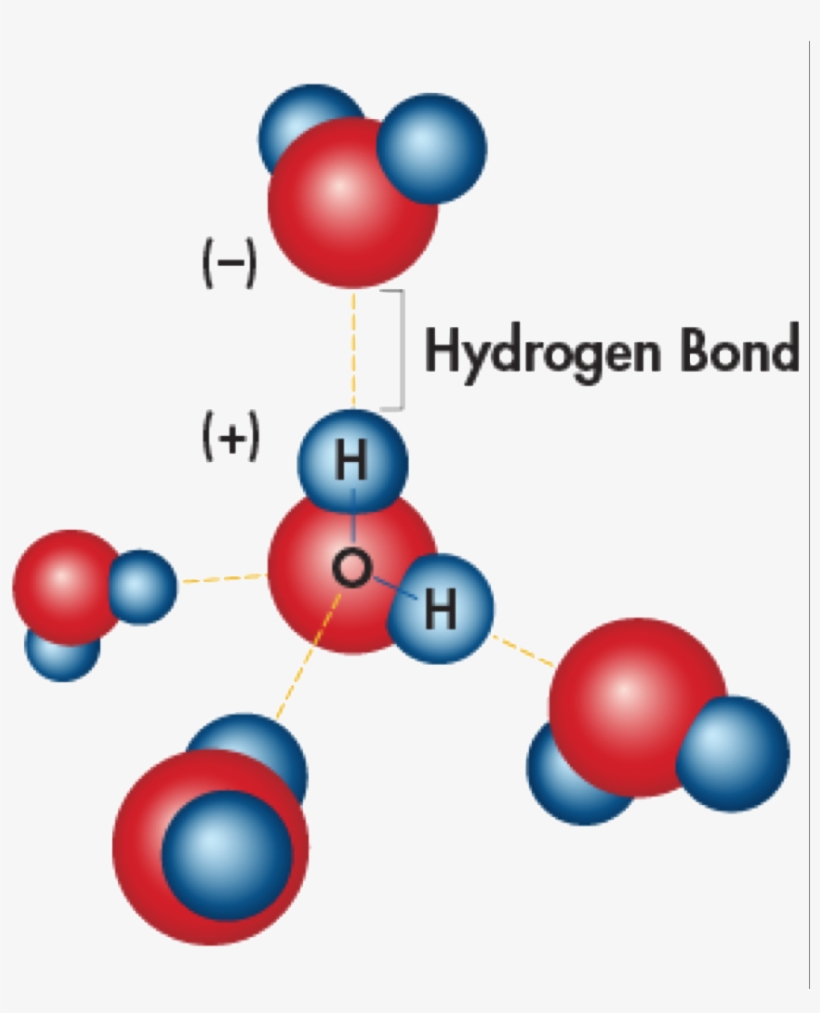
High Surface Tension Of Hydrogen Bonding . Next to mercury, water has the highest surface tension of all commonly occurring liquids. The water strider takes advantage of the fact that the water surface acts like an elastic film that resists deformation when a small weight is placed on it. Hydrogen bonding between water molecules (intermolecular forces) explains. Because of the relatively high attraction of water molecules to each other through a web of hydrogen bonds, water has a higher surface. Surface tension is a manifestation of the presence of. Water has high surface tension, which can be explained by its polarity and hydrogen bonding. Water has a high surface tension. (if you are careful, you can also. Compared to most other liquids, water also has a high surface tension. Surface tension is the ability of a liquid surface to resist any external forces. Water consists of one oxygen atom flanked by two hydrogen atoms. Have you ever watched an insect walk across the surface of a pond?
from mungfali.com
The water strider takes advantage of the fact that the water surface acts like an elastic film that resists deformation when a small weight is placed on it. Hydrogen bonding between water molecules (intermolecular forces) explains. Surface tension is the ability of a liquid surface to resist any external forces. Surface tension is a manifestation of the presence of. Compared to most other liquids, water also has a high surface tension. Next to mercury, water has the highest surface tension of all commonly occurring liquids. Water consists of one oxygen atom flanked by two hydrogen atoms. Have you ever watched an insect walk across the surface of a pond? Water has a high surface tension. Because of the relatively high attraction of water molecules to each other through a web of hydrogen bonds, water has a higher surface.
Hydrogen Bonding Forces
High Surface Tension Of Hydrogen Bonding Water has high surface tension, which can be explained by its polarity and hydrogen bonding. Surface tension is the ability of a liquid surface to resist any external forces. Compared to most other liquids, water also has a high surface tension. Water has high surface tension, which can be explained by its polarity and hydrogen bonding. Hydrogen bonding between water molecules (intermolecular forces) explains. (if you are careful, you can also. Water has a high surface tension. The water strider takes advantage of the fact that the water surface acts like an elastic film that resists deformation when a small weight is placed on it. Because of the relatively high attraction of water molecules to each other through a web of hydrogen bonds, water has a higher surface. Surface tension is a manifestation of the presence of. Next to mercury, water has the highest surface tension of all commonly occurring liquids. Water consists of one oxygen atom flanked by two hydrogen atoms. Have you ever watched an insect walk across the surface of a pond?
From www.youtube.com
Surface Tension of Water Explained YouTube High Surface Tension Of Hydrogen Bonding Because of the relatively high attraction of water molecules to each other through a web of hydrogen bonds, water has a higher surface. Water has a high surface tension. Compared to most other liquids, water also has a high surface tension. Next to mercury, water has the highest surface tension of all commonly occurring liquids. (if you are careful, you. High Surface Tension Of Hydrogen Bonding.
From www.scienceabc.com
Surface Tension Definition, Explanation, Examples And Significance High Surface Tension Of Hydrogen Bonding Hydrogen bonding between water molecules (intermolecular forces) explains. (if you are careful, you can also. Surface tension is a manifestation of the presence of. Because of the relatively high attraction of water molecules to each other through a web of hydrogen bonds, water has a higher surface. Water consists of one oxygen atom flanked by two hydrogen atoms. Water has. High Surface Tension Of Hydrogen Bonding.
From www.slideserve.com
PPT CHEMISTRY Concepts and connections Ch. 2 PowerPoint Presentation High Surface Tension Of Hydrogen Bonding Water has high surface tension, which can be explained by its polarity and hydrogen bonding. Surface tension is a manifestation of the presence of. Water consists of one oxygen atom flanked by two hydrogen atoms. Hydrogen bonding between water molecules (intermolecular forces) explains. Because of the relatively high attraction of water molecules to each other through a web of hydrogen. High Surface Tension Of Hydrogen Bonding.
From slidetodoc.com
POLARITY INTERMOLECULAR FORCES HYBRIDIZATION Molecular Polarity The uneven High Surface Tension Of Hydrogen Bonding Water consists of one oxygen atom flanked by two hydrogen atoms. Compared to most other liquids, water also has a high surface tension. The water strider takes advantage of the fact that the water surface acts like an elastic film that resists deformation when a small weight is placed on it. Next to mercury, water has the highest surface tension. High Surface Tension Of Hydrogen Bonding.
From mungfali.com
Hydrogen Bonding Forces High Surface Tension Of Hydrogen Bonding Surface tension is the ability of a liquid surface to resist any external forces. Water has high surface tension, which can be explained by its polarity and hydrogen bonding. Water has a high surface tension. Compared to most other liquids, water also has a high surface tension. The water strider takes advantage of the fact that the water surface acts. High Surface Tension Of Hydrogen Bonding.
From ar.inspiredpencil.com
The Relationship Between Hydrogen Bonding And Surface Tension, Adhesion High Surface Tension Of Hydrogen Bonding The water strider takes advantage of the fact that the water surface acts like an elastic film that resists deformation when a small weight is placed on it. Surface tension is the ability of a liquid surface to resist any external forces. Compared to most other liquids, water also has a high surface tension. (if you are careful, you can. High Surface Tension Of Hydrogen Bonding.
From dokumen.tips
(PPTX) Water and Aqueous Systems Chapter 17. Objectives 1.Describe the High Surface Tension Of Hydrogen Bonding The water strider takes advantage of the fact that the water surface acts like an elastic film that resists deformation when a small weight is placed on it. Because of the relatively high attraction of water molecules to each other through a web of hydrogen bonds, water has a higher surface. Water has a high surface tension. Water consists of. High Surface Tension Of Hydrogen Bonding.
From slideplayer.com
Chapter 15 “Water and Aqueous Systems” ppt download High Surface Tension Of Hydrogen Bonding Because of the relatively high attraction of water molecules to each other through a web of hydrogen bonds, water has a higher surface. Water consists of one oxygen atom flanked by two hydrogen atoms. Next to mercury, water has the highest surface tension of all commonly occurring liquids. The water strider takes advantage of the fact that the water surface. High Surface Tension Of Hydrogen Bonding.
From www.slideserve.com
PPT Hydrogen Bonding PowerPoint Presentation, free download ID297441 High Surface Tension Of Hydrogen Bonding The water strider takes advantage of the fact that the water surface acts like an elastic film that resists deformation when a small weight is placed on it. Water has a high surface tension. Next to mercury, water has the highest surface tension of all commonly occurring liquids. (if you are careful, you can also. Hydrogen bonding between water molecules. High Surface Tension Of Hydrogen Bonding.
From philschatz.com
Chemical Bonds · Anatomy and Physiology High Surface Tension Of Hydrogen Bonding Surface tension is the ability of a liquid surface to resist any external forces. Next to mercury, water has the highest surface tension of all commonly occurring liquids. The water strider takes advantage of the fact that the water surface acts like an elastic film that resists deformation when a small weight is placed on it. (if you are careful,. High Surface Tension Of Hydrogen Bonding.
From www.expii.com
Hydrogen Bonds — Overview & Examples Expii High Surface Tension Of Hydrogen Bonding Have you ever watched an insect walk across the surface of a pond? Because of the relatively high attraction of water molecules to each other through a web of hydrogen bonds, water has a higher surface. Next to mercury, water has the highest surface tension of all commonly occurring liquids. Compared to most other liquids, water also has a high. High Surface Tension Of Hydrogen Bonding.
From www.biolo1100.nicerweb.com
surface_tension/02_13 High Surface Tension Of Hydrogen Bonding Surface tension is a manifestation of the presence of. Hydrogen bonding between water molecules (intermolecular forces) explains. Surface tension is the ability of a liquid surface to resist any external forces. Compared to most other liquids, water also has a high surface tension. (if you are careful, you can also. The water strider takes advantage of the fact that the. High Surface Tension Of Hydrogen Bonding.
From errantscience.com
Water surface tension ErrantScience High Surface Tension Of Hydrogen Bonding The water strider takes advantage of the fact that the water surface acts like an elastic film that resists deformation when a small weight is placed on it. (if you are careful, you can also. Water consists of one oxygen atom flanked by two hydrogen atoms. Water has a high surface tension. Compared to most other liquids, water also has. High Surface Tension Of Hydrogen Bonding.
From www.slideserve.com
PPT Chpt 16 Mixtures PowerPoint Presentation, free download ID4611101 High Surface Tension Of Hydrogen Bonding The water strider takes advantage of the fact that the water surface acts like an elastic film that resists deformation when a small weight is placed on it. Surface tension is the ability of a liquid surface to resist any external forces. Because of the relatively high attraction of water molecules to each other through a web of hydrogen bonds,. High Surface Tension Of Hydrogen Bonding.
From www.slideserve.com
PPT Ch. 17 and Ch. 18 Water and Aqueous Solutions PowerPoint High Surface Tension Of Hydrogen Bonding Hydrogen bonding between water molecules (intermolecular forces) explains. The water strider takes advantage of the fact that the water surface acts like an elastic film that resists deformation when a small weight is placed on it. Compared to most other liquids, water also has a high surface tension. Surface tension is the ability of a liquid surface to resist any. High Surface Tension Of Hydrogen Bonding.
From www.slideserve.com
PPT Limnology! PowerPoint Presentation, free download ID1426315 High Surface Tension Of Hydrogen Bonding Water consists of one oxygen atom flanked by two hydrogen atoms. Compared to most other liquids, water also has a high surface tension. Because of the relatively high attraction of water molecules to each other through a web of hydrogen bonds, water has a higher surface. (if you are careful, you can also. The water strider takes advantage of the. High Surface Tension Of Hydrogen Bonding.
From present5.com
Water s Special Properties Hydrogen Bonding Surface Tension and High Surface Tension Of Hydrogen Bonding Water has high surface tension, which can be explained by its polarity and hydrogen bonding. Because of the relatively high attraction of water molecules to each other through a web of hydrogen bonds, water has a higher surface. Hydrogen bonding between water molecules (intermolecular forces) explains. Next to mercury, water has the highest surface tension of all commonly occurring liquids.. High Surface Tension Of Hydrogen Bonding.
From www.slideserve.com
PPT Chapter 3 Water and Life PowerPoint Presentation, free download High Surface Tension Of Hydrogen Bonding Surface tension is the ability of a liquid surface to resist any external forces. (if you are careful, you can also. Water has a high surface tension. Water has high surface tension, which can be explained by its polarity and hydrogen bonding. Surface tension is a manifestation of the presence of. Water consists of one oxygen atom flanked by two. High Surface Tension Of Hydrogen Bonding.
From www.youtube.com
Surface Tension and Hydrogen Bonding Demonstration YouTube High Surface Tension Of Hydrogen Bonding Water has a high surface tension. (if you are careful, you can also. Water consists of one oxygen atom flanked by two hydrogen atoms. Have you ever watched an insect walk across the surface of a pond? Surface tension is the ability of a liquid surface to resist any external forces. Next to mercury, water has the highest surface tension. High Surface Tension Of Hydrogen Bonding.
From www.slideshare.net
Water, Hydrogen Bonds, and the Hydrologic Cycle High Surface Tension Of Hydrogen Bonding Have you ever watched an insect walk across the surface of a pond? Water has high surface tension, which can be explained by its polarity and hydrogen bonding. Next to mercury, water has the highest surface tension of all commonly occurring liquids. Because of the relatively high attraction of water molecules to each other through a web of hydrogen bonds,. High Surface Tension Of Hydrogen Bonding.
From www.slideserve.com
PPT Chapter 15 “Water and Aqueous Systems” PowerPoint Presentation High Surface Tension Of Hydrogen Bonding Compared to most other liquids, water also has a high surface tension. The water strider takes advantage of the fact that the water surface acts like an elastic film that resists deformation when a small weight is placed on it. Surface tension is the ability of a liquid surface to resist any external forces. Have you ever watched an insect. High Surface Tension Of Hydrogen Bonding.
From www.numerade.com
SOLVED All of the following are properties of water EXCEPT It is a High Surface Tension Of Hydrogen Bonding (if you are careful, you can also. Water has a high surface tension. Surface tension is the ability of a liquid surface to resist any external forces. Hydrogen bonding between water molecules (intermolecular forces) explains. Because of the relatively high attraction of water molecules to each other through a web of hydrogen bonds, water has a higher surface. Surface tension. High Surface Tension Of Hydrogen Bonding.
From www.slideserve.com
PPT Types of Bonding Water Molecule Formation & Configuration Unusual High Surface Tension Of Hydrogen Bonding Have you ever watched an insect walk across the surface of a pond? Hydrogen bonding between water molecules (intermolecular forces) explains. Water has a high surface tension. (if you are careful, you can also. Compared to most other liquids, water also has a high surface tension. Surface tension is the ability of a liquid surface to resist any external forces.. High Surface Tension Of Hydrogen Bonding.
From www.slideserve.com
PPT Chapter 11 Intermolecular Forces, Liquids, and Solids PowerPoint High Surface Tension Of Hydrogen Bonding Next to mercury, water has the highest surface tension of all commonly occurring liquids. Because of the relatively high attraction of water molecules to each other through a web of hydrogen bonds, water has a higher surface. Hydrogen bonding between water molecules (intermolecular forces) explains. Compared to most other liquids, water also has a high surface tension. The water strider. High Surface Tension Of Hydrogen Bonding.
From slideplayer.com
Water and Aqueous Systems ppt download High Surface Tension Of Hydrogen Bonding Surface tension is the ability of a liquid surface to resist any external forces. Surface tension is a manifestation of the presence of. (if you are careful, you can also. Hydrogen bonding between water molecules (intermolecular forces) explains. Because of the relatively high attraction of water molecules to each other through a web of hydrogen bonds, water has a higher. High Surface Tension Of Hydrogen Bonding.
From www.savemyexams.com
Hydrogen Bonding Edexcel A Level Chemistry Revision Notes 2017 High Surface Tension Of Hydrogen Bonding The water strider takes advantage of the fact that the water surface acts like an elastic film that resists deformation when a small weight is placed on it. Water has high surface tension, which can be explained by its polarity and hydrogen bonding. Compared to most other liquids, water also has a high surface tension. Water consists of one oxygen. High Surface Tension Of Hydrogen Bonding.
From ideal.accelerate-ed.com
Surface Tension High Surface Tension Of Hydrogen Bonding Hydrogen bonding between water molecules (intermolecular forces) explains. (if you are careful, you can also. Surface tension is a manifestation of the presence of. The water strider takes advantage of the fact that the water surface acts like an elastic film that resists deformation when a small weight is placed on it. Water has high surface tension, which can be. High Surface Tension Of Hydrogen Bonding.
From www.slideserve.com
PPT Chapter 2 PowerPoint Presentation, free download ID5430343 High Surface Tension Of Hydrogen Bonding Hydrogen bonding between water molecules (intermolecular forces) explains. Because of the relatively high attraction of water molecules to each other through a web of hydrogen bonds, water has a higher surface. Surface tension is the ability of a liquid surface to resist any external forces. Water has high surface tension, which can be explained by its polarity and hydrogen bonding.. High Surface Tension Of Hydrogen Bonding.
From www.shutterstock.com
Hydrogen Bonds Surface Tension 库存矢量图(免版税)1640032501 High Surface Tension Of Hydrogen Bonding Surface tension is a manifestation of the presence of. Water has high surface tension, which can be explained by its polarity and hydrogen bonding. Surface tension is the ability of a liquid surface to resist any external forces. Have you ever watched an insect walk across the surface of a pond? Next to mercury, water has the highest surface tension. High Surface Tension Of Hydrogen Bonding.
From slideplayer.com
Hydrogen Bonding and The Nature of Solutions ppt download High Surface Tension Of Hydrogen Bonding Water has high surface tension, which can be explained by its polarity and hydrogen bonding. Next to mercury, water has the highest surface tension of all commonly occurring liquids. Surface tension is the ability of a liquid surface to resist any external forces. Because of the relatively high attraction of water molecules to each other through a web of hydrogen. High Surface Tension Of Hydrogen Bonding.
From chem.libretexts.org
1.11 The Bonds in Water Chemistry LibreTexts High Surface Tension Of Hydrogen Bonding Next to mercury, water has the highest surface tension of all commonly occurring liquids. Hydrogen bonding between water molecules (intermolecular forces) explains. Water has a high surface tension. Compared to most other liquids, water also has a high surface tension. Have you ever watched an insect walk across the surface of a pond? Surface tension is a manifestation of the. High Surface Tension Of Hydrogen Bonding.
From www.numerade.com
SOLVED What property of water allows for the high surface tension of High Surface Tension Of Hydrogen Bonding Water has a high surface tension. Hydrogen bonding between water molecules (intermolecular forces) explains. Water has high surface tension, which can be explained by its polarity and hydrogen bonding. Have you ever watched an insect walk across the surface of a pond? Because of the relatively high attraction of water molecules to each other through a web of hydrogen bonds,. High Surface Tension Of Hydrogen Bonding.
From universe-review.ca
Molecules High Surface Tension Of Hydrogen Bonding Because of the relatively high attraction of water molecules to each other through a web of hydrogen bonds, water has a higher surface. Compared to most other liquids, water also has a high surface tension. The water strider takes advantage of the fact that the water surface acts like an elastic film that resists deformation when a small weight is. High Surface Tension Of Hydrogen Bonding.
From slideplayer.com
Chapter 5 Gases,Liquids, and Solids ppt download High Surface Tension Of Hydrogen Bonding Water has a high surface tension. Because of the relatively high attraction of water molecules to each other through a web of hydrogen bonds, water has a higher surface. The water strider takes advantage of the fact that the water surface acts like an elastic film that resists deformation when a small weight is placed on it. Compared to most. High Surface Tension Of Hydrogen Bonding.
From slideplayer.com
Intermolecular Forces (IMF) ppt download High Surface Tension Of Hydrogen Bonding Compared to most other liquids, water also has a high surface tension. Surface tension is the ability of a liquid surface to resist any external forces. (if you are careful, you can also. Water has a high surface tension. The water strider takes advantage of the fact that the water surface acts like an elastic film that resists deformation when. High Surface Tension Of Hydrogen Bonding.