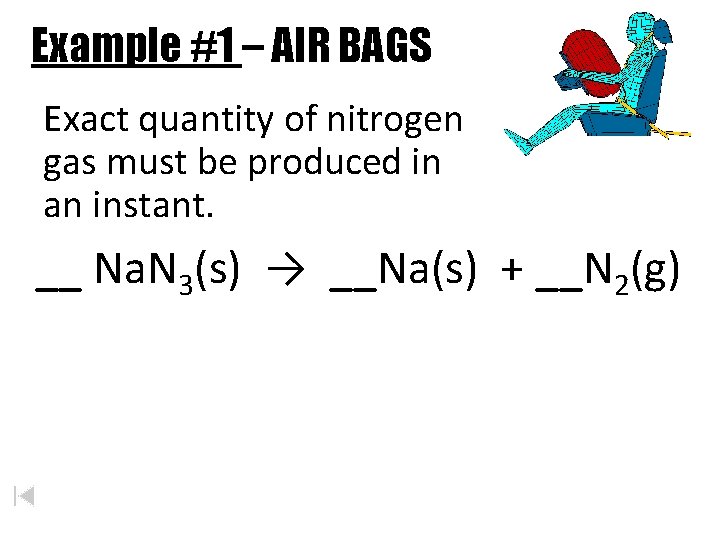Airbags And Stoichiometry . explain the concept of stoichiometry as it pertains to chemical reactions; Stoichiometry and the gas constant experiment. air bags are not inflated from some compressed gas source but rather from the products of a chemical reaction. Rachel casiday and regina frey. Guanidinium nitrate, plus a copper nitrate oxidizer. In case of a collision, a reaction is triggered so that the rapid decomposition of sodium azide produces nitrogen gas, filling the air bag. today’s airbags use a different chemical to produce nitrogen gas: Cars and many other vehicles have air bags in them. in this lab, you will conduct an experiment where you will design and construct an airbag by reacting an acid and a base. explain the concept of stoichiometry as it pertains to chemical reactions; gas laws save lives: how much azide is needed to fill an air bag? after using the ideal gas law to predict the moles of gas required to fill the ‘airbag’, you can then use simple stoichiometry to. Use balanced chemical equations to derive stoichiometric factors.
from slidetodoc.com
Stoichiometry and the gas constant experiment. air bags are not inflated from some compressed gas source but rather from the products of a chemical reaction. Guanidinium nitrate, plus a copper nitrate oxidizer. gas laws save lives: explain the concept of stoichiometry as it pertains to chemical reactions; Rachel casiday and regina frey. In case of a collision, a reaction is triggered so that the rapid decomposition of sodium azide produces nitrogen gas, filling the air bag. how much azide is needed to fill an air bag? after using the ideal gas law to predict the moles of gas required to fill the ‘airbag’, you can then use simple stoichiometry to. in this lab, you will conduct an experiment where you will design and construct an airbag by reacting an acid and a base.
Real Life Stoichiometry Examples Example 1 AIR BAGS
Airbags And Stoichiometry how much azide is needed to fill an air bag? how much azide is needed to fill an air bag? today’s airbags use a different chemical to produce nitrogen gas: explain the concept of stoichiometry as it pertains to chemical reactions; Cars and many other vehicles have air bags in them. Stoichiometry and the gas constant experiment. Guanidinium nitrate, plus a copper nitrate oxidizer. in this lab, you will conduct an experiment where you will design and construct an airbag by reacting an acid and a base. In case of a collision, a reaction is triggered so that the rapid decomposition of sodium azide produces nitrogen gas, filling the air bag. Use balanced chemical equations to derive stoichiometric factors. after using the ideal gas law to predict the moles of gas required to fill the ‘airbag’, you can then use simple stoichiometry to. explain the concept of stoichiometry as it pertains to chemical reactions; Rachel casiday and regina frey. air bags are not inflated from some compressed gas source but rather from the products of a chemical reaction. gas laws save lives:
From www.numerade.com
SOLVED Ideal Gas Stoichiometry Example The of sodium Airbags And Stoichiometry Cars and many other vehicles have air bags in them. Stoichiometry and the gas constant experiment. how much azide is needed to fill an air bag? Use balanced chemical equations to derive stoichiometric factors. gas laws save lives: in this lab, you will conduct an experiment where you will design and construct an airbag by reacting an. Airbags And Stoichiometry.
From studylib.net
Lab Airbag Stoichiometry Airbags And Stoichiometry Cars and many other vehicles have air bags in them. Guanidinium nitrate, plus a copper nitrate oxidizer. how much azide is needed to fill an air bag? air bags are not inflated from some compressed gas source but rather from the products of a chemical reaction. Stoichiometry and the gas constant experiment. explain the concept of stoichiometry. Airbags And Stoichiometry.
From www.scribd.com
10 Air Bags Stoich PDF Airbag Stoichiometry Airbags And Stoichiometry Guanidinium nitrate, plus a copper nitrate oxidizer. in this lab, you will conduct an experiment where you will design and construct an airbag by reacting an acid and a base. after using the ideal gas law to predict the moles of gas required to fill the ‘airbag’, you can then use simple stoichiometry to. Stoichiometry and the gas. Airbags And Stoichiometry.
From www.chegg.com
Solved Stoichiometry of Airbags When automobile airbags are Airbags And Stoichiometry after using the ideal gas law to predict the moles of gas required to fill the ‘airbag’, you can then use simple stoichiometry to. gas laws save lives: how much azide is needed to fill an air bag? Cars and many other vehicles have air bags in them. explain the concept of stoichiometry as it pertains. Airbags And Stoichiometry.
From www.lessonplanet.com
Gas Stoichiometry How Does an Airbag Work? Instructional Video for 9th Airbags And Stoichiometry today’s airbags use a different chemical to produce nitrogen gas: In case of a collision, a reaction is triggered so that the rapid decomposition of sodium azide produces nitrogen gas, filling the air bag. explain the concept of stoichiometry as it pertains to chemical reactions; Use balanced chemical equations to derive stoichiometric factors. Stoichiometry and the gas constant. Airbags And Stoichiometry.
From www.slideserve.com
PPT quest Stoichiometry and Air Bags qlink.queensu PowerPoint Airbags And Stoichiometry Guanidinium nitrate, plus a copper nitrate oxidizer. Rachel casiday and regina frey. Stoichiometry and the gas constant experiment. in this lab, you will conduct an experiment where you will design and construct an airbag by reacting an acid and a base. gas laws save lives: Use balanced chemical equations to derive stoichiometric factors. explain the concept of. Airbags And Stoichiometry.
From slidetodoc.com
Stoichiometry in the Real World Air Bag Design Airbags And Stoichiometry Guanidinium nitrate, plus a copper nitrate oxidizer. explain the concept of stoichiometry as it pertains to chemical reactions; Stoichiometry and the gas constant experiment. gas laws save lives: today’s airbags use a different chemical to produce nitrogen gas: in this lab, you will conduct an experiment where you will design and construct an airbag by reacting. Airbags And Stoichiometry.
From www.slideserve.com
PPT Gas Stoichiometry! PowerPoint Presentation, free download ID Airbags And Stoichiometry Rachel casiday and regina frey. Cars and many other vehicles have air bags in them. after using the ideal gas law to predict the moles of gas required to fill the ‘airbag’, you can then use simple stoichiometry to. in this lab, you will conduct an experiment where you will design and construct an airbag by reacting an. Airbags And Stoichiometry.
From slideplayer.com
Chapter 5 Gases. ppt download Airbags And Stoichiometry in this lab, you will conduct an experiment where you will design and construct an airbag by reacting an acid and a base. Stoichiometry and the gas constant experiment. explain the concept of stoichiometry as it pertains to chemical reactions; today’s airbags use a different chemical to produce nitrogen gas: Guanidinium nitrate, plus a copper nitrate oxidizer.. Airbags And Stoichiometry.
From oneclass.com
OneClass Gas Stoichiometry Automobile Airbags 6. Calculate the volume Airbags And Stoichiometry Use balanced chemical equations to derive stoichiometric factors. in this lab, you will conduct an experiment where you will design and construct an airbag by reacting an acid and a base. Guanidinium nitrate, plus a copper nitrate oxidizer. explain the concept of stoichiometry as it pertains to chemical reactions; Cars and many other vehicles have air bags in. Airbags And Stoichiometry.
From slideplayer.com
How to Use This Presentation ppt download Airbags And Stoichiometry after using the ideal gas law to predict the moles of gas required to fill the ‘airbag’, you can then use simple stoichiometry to. Guanidinium nitrate, plus a copper nitrate oxidizer. how much azide is needed to fill an air bag? explain the concept of stoichiometry as it pertains to chemical reactions; in this lab, you. Airbags And Stoichiometry.
From www.youtube.com
Airbag Challenge Stoichiometry YouTube Airbags And Stoichiometry in this lab, you will conduct an experiment where you will design and construct an airbag by reacting an acid and a base. Cars and many other vehicles have air bags in them. explain the concept of stoichiometry as it pertains to chemical reactions; Guanidinium nitrate, plus a copper nitrate oxidizer. Rachel casiday and regina frey. after. Airbags And Stoichiometry.
From present5.com
Stoichiometry in the Real World Air Bag Airbags And Stoichiometry today’s airbags use a different chemical to produce nitrogen gas: explain the concept of stoichiometry as it pertains to chemical reactions; Cars and many other vehicles have air bags in them. Use balanced chemical equations to derive stoichiometric factors. In case of a collision, a reaction is triggered so that the rapid decomposition of sodium azide produces nitrogen. Airbags And Stoichiometry.
From www.scribd.com
Airbags Design 7 Stoichiometry Examples in Real Life PDF Airbags And Stoichiometry Guanidinium nitrate, plus a copper nitrate oxidizer. how much azide is needed to fill an air bag? Cars and many other vehicles have air bags in them. explain the concept of stoichiometry as it pertains to chemical reactions; in this lab, you will conduct an experiment where you will design and construct an airbag by reacting an. Airbags And Stoichiometry.
From slidetodoc.com
Stoichiometry in the Real World Air Bag Design Airbags And Stoichiometry today’s airbags use a different chemical to produce nitrogen gas: explain the concept of stoichiometry as it pertains to chemical reactions; In case of a collision, a reaction is triggered so that the rapid decomposition of sodium azide produces nitrogen gas, filling the air bag. Rachel casiday and regina frey. after using the ideal gas law to. Airbags And Stoichiometry.
From www.scribd.com
Airbags PDF Airbag Stoichiometry Airbags And Stoichiometry Rachel casiday and regina frey. Use balanced chemical equations to derive stoichiometric factors. today’s airbags use a different chemical to produce nitrogen gas: after using the ideal gas law to predict the moles of gas required to fill the ‘airbag’, you can then use simple stoichiometry to. air bags are not inflated from some compressed gas source. Airbags And Stoichiometry.
From slidetodoc.com
Real Life Stoichiometry Examples Example 1 AIR BAGS Airbags And Stoichiometry Cars and many other vehicles have air bags in them. after using the ideal gas law to predict the moles of gas required to fill the ‘airbag’, you can then use simple stoichiometry to. gas laws save lives: explain the concept of stoichiometry as it pertains to chemical reactions; in this lab, you will conduct an. Airbags And Stoichiometry.
From slidetodoc.com
Stoichiometry in the Real World Air Bag Design Airbags And Stoichiometry after using the ideal gas law to predict the moles of gas required to fill the ‘airbag’, you can then use simple stoichiometry to. how much azide is needed to fill an air bag? gas laws save lives: in this lab, you will conduct an experiment where you will design and construct an airbag by reacting. Airbags And Stoichiometry.
From slidetodoc.com
Real Life Stoichiometry Examples Example 1 AIR BAGS Airbags And Stoichiometry Stoichiometry and the gas constant experiment. Cars and many other vehicles have air bags in them. gas laws save lives: in this lab, you will conduct an experiment where you will design and construct an airbag by reacting an acid and a base. explain the concept of stoichiometry as it pertains to chemical reactions; Guanidinium nitrate, plus. Airbags And Stoichiometry.
From slideplayer.com
How to Use This Presentation ppt download Airbags And Stoichiometry in this lab, you will conduct an experiment where you will design and construct an airbag by reacting an acid and a base. In case of a collision, a reaction is triggered so that the rapid decomposition of sodium azide produces nitrogen gas, filling the air bag. explain the concept of stoichiometry as it pertains to chemical reactions;. Airbags And Stoichiometry.
From www.chegg.com
Solved CHM 111 Stoichiometry in the Real World Airbags Name Airbags And Stoichiometry Use balanced chemical equations to derive stoichiometric factors. in this lab, you will conduct an experiment where you will design and construct an airbag by reacting an acid and a base. Rachel casiday and regina frey. Stoichiometry and the gas constant experiment. explain the concept of stoichiometry as it pertains to chemical reactions; Cars and many other vehicles. Airbags And Stoichiometry.
From www.studocu.com
Airbags exp ccc Stoichiometry Baking Soda and Vinegar Reactions Airbags And Stoichiometry Rachel casiday and regina frey. Use balanced chemical equations to derive stoichiometric factors. explain the concept of stoichiometry as it pertains to chemical reactions; Guanidinium nitrate, plus a copper nitrate oxidizer. today’s airbags use a different chemical to produce nitrogen gas: how much azide is needed to fill an air bag? after using the ideal gas. Airbags And Stoichiometry.
From www.nsta.org
Airbags as RealLife Applications for Science NSTA Airbags And Stoichiometry today’s airbags use a different chemical to produce nitrogen gas: in this lab, you will conduct an experiment where you will design and construct an airbag by reacting an acid and a base. Stoichiometry and the gas constant experiment. how much azide is needed to fill an air bag? In case of a collision, a reaction is. Airbags And Stoichiometry.
From slidetodoc.com
Real Life Stoichiometry Examples Example 1 AIR BAGS Airbags And Stoichiometry today’s airbags use a different chemical to produce nitrogen gas: Rachel casiday and regina frey. explain the concept of stoichiometry as it pertains to chemical reactions; how much azide is needed to fill an air bag? Cars and many other vehicles have air bags in them. after using the ideal gas law to predict the moles. Airbags And Stoichiometry.
From slidetodoc.com
Real Life Stoichiometry Examples Example 1 AIR BAGS Airbags And Stoichiometry Cars and many other vehicles have air bags in them. after using the ideal gas law to predict the moles of gas required to fill the ‘airbag’, you can then use simple stoichiometry to. Guanidinium nitrate, plus a copper nitrate oxidizer. Stoichiometry and the gas constant experiment. explain the concept of stoichiometry as it pertains to chemical reactions;. Airbags And Stoichiometry.
From slideplayer.com
Ch 9 Stoichiometry “equal measure” ppt download Airbags And Stoichiometry In case of a collision, a reaction is triggered so that the rapid decomposition of sodium azide produces nitrogen gas, filling the air bag. Guanidinium nitrate, plus a copper nitrate oxidizer. Stoichiometry and the gas constant experiment. how much azide is needed to fill an air bag? gas laws save lives: Rachel casiday and regina frey. explain. Airbags And Stoichiometry.
From www.youtube.com
Stoichiometry with Airbags YouTube Airbags And Stoichiometry Stoichiometry and the gas constant experiment. after using the ideal gas law to predict the moles of gas required to fill the ‘airbag’, you can then use simple stoichiometry to. how much azide is needed to fill an air bag? air bags are not inflated from some compressed gas source but rather from the products of a. Airbags And Stoichiometry.
From www.youtube.com
Gas Stoichiometry How does an airbag work? YouTube Airbags And Stoichiometry air bags are not inflated from some compressed gas source but rather from the products of a chemical reaction. In case of a collision, a reaction is triggered so that the rapid decomposition of sodium azide produces nitrogen gas, filling the air bag. Use balanced chemical equations to derive stoichiometric factors. today’s airbags use a different chemical to. Airbags And Stoichiometry.
From slideplayer.com
Unit 8 Gases. ppt download Airbags And Stoichiometry Rachel casiday and regina frey. Stoichiometry and the gas constant experiment. explain the concept of stoichiometry as it pertains to chemical reactions; after using the ideal gas law to predict the moles of gas required to fill the ‘airbag’, you can then use simple stoichiometry to. Use balanced chemical equations to derive stoichiometric factors. Guanidinium nitrate, plus a. Airbags And Stoichiometry.
From www.scribd.com
Airbags and Stoichiometry Answers PDF Airbag Chemical Substances Airbags And Stoichiometry gas laws save lives: after using the ideal gas law to predict the moles of gas required to fill the ‘airbag’, you can then use simple stoichiometry to. Cars and many other vehicles have air bags in them. explain the concept of stoichiometry as it pertains to chemical reactions; Guanidinium nitrate, plus a copper nitrate oxidizer. . Airbags And Stoichiometry.
From www.scribd.com
Air Bags! Stoichiometry That Saves Lives! Lembar Kerja 1 PDF Airbags And Stoichiometry today’s airbags use a different chemical to produce nitrogen gas: In case of a collision, a reaction is triggered so that the rapid decomposition of sodium azide produces nitrogen gas, filling the air bag. explain the concept of stoichiometry as it pertains to chemical reactions; Stoichiometry and the gas constant experiment. Use balanced chemical equations to derive stoichiometric. Airbags And Stoichiometry.
From www.numerade.com
SOLVED Ideal Gas Stoichiometry Example The of sodium Airbags And Stoichiometry explain the concept of stoichiometry as it pertains to chemical reactions; Guanidinium nitrate, plus a copper nitrate oxidizer. In case of a collision, a reaction is triggered so that the rapid decomposition of sodium azide produces nitrogen gas, filling the air bag. air bags are not inflated from some compressed gas source but rather from the products of. Airbags And Stoichiometry.
From www.pdffiller.com
Fillable Online Stoichiometry and Safety Air Bags Fax Email Print Airbags And Stoichiometry in this lab, you will conduct an experiment where you will design and construct an airbag by reacting an acid and a base. explain the concept of stoichiometry as it pertains to chemical reactions; Guanidinium nitrate, plus a copper nitrate oxidizer. Rachel casiday and regina frey. Cars and many other vehicles have air bags in them. Use balanced. Airbags And Stoichiometry.
From docs.google.com
Air Bags & Stoichiometry Lab Google Docs Airbags And Stoichiometry Guanidinium nitrate, plus a copper nitrate oxidizer. how much azide is needed to fill an air bag? explain the concept of stoichiometry as it pertains to chemical reactions; today’s airbags use a different chemical to produce nitrogen gas: air bags are not inflated from some compressed gas source but rather from the products of a chemical. Airbags And Stoichiometry.
From slideplayer.com
How to Use This Presentation ppt download Airbags And Stoichiometry In case of a collision, a reaction is triggered so that the rapid decomposition of sodium azide produces nitrogen gas, filling the air bag. air bags are not inflated from some compressed gas source but rather from the products of a chemical reaction. Stoichiometry and the gas constant experiment. Guanidinium nitrate, plus a copper nitrate oxidizer. Use balanced chemical. Airbags And Stoichiometry.