Standard Enthalpy Of Formation Formula . the standard enthalpy of formation of any element in its standard state is zero by definition. a standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is. See examples, applications and practice problems. the standard enthalpy of formation, \(δh^\circ_\ce{f}\), is the enthalpy change accompanying the formation of 1. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of. the standard enthalpy of formation, δh o f, is the enthalpy change for a formation equation when all substances are in their standard. the standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created. For example, although oxygen can exist as ozone (o 3 ), atomic. learn the definition, formula and measurement of standard enthalpy of formation, the enthalpy change when 1 mol of a substance is formed from its elements.
from www.chegg.com
For example, although oxygen can exist as ozone (o 3 ), atomic. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of. See examples, applications and practice problems. a standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is. the standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created. learn the definition, formula and measurement of standard enthalpy of formation, the enthalpy change when 1 mol of a substance is formed from its elements. the standard enthalpy of formation of any element in its standard state is zero by definition. the standard enthalpy of formation, δh o f, is the enthalpy change for a formation equation when all substances are in their standard. the standard enthalpy of formation, \(δh^\circ_\ce{f}\), is the enthalpy change accompanying the formation of 1.
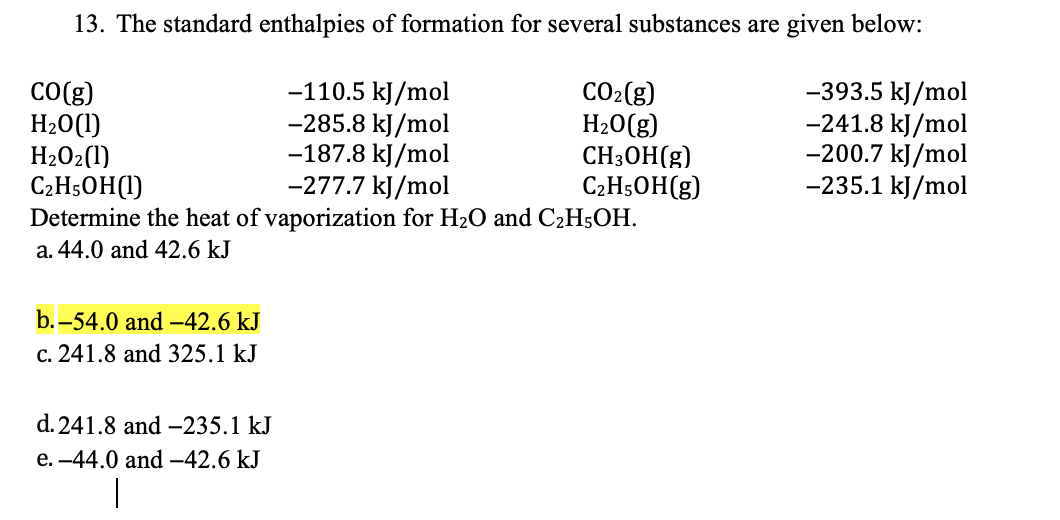
Solved 13. The standard enthalpies of formation for several
Standard Enthalpy Of Formation Formula For example, although oxygen can exist as ozone (o 3 ), atomic. the standard enthalpy of formation of any element in its standard state is zero by definition. See examples, applications and practice problems. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of. For example, although oxygen can exist as ozone (o 3 ), atomic. the standard enthalpy of formation, \(δh^\circ_\ce{f}\), is the enthalpy change accompanying the formation of 1. the standard enthalpy of formation, δh o f, is the enthalpy change for a formation equation when all substances are in their standard. the standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created. a standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is. learn the definition, formula and measurement of standard enthalpy of formation, the enthalpy change when 1 mol of a substance is formed from its elements.
From www.slideserve.com
PPT STANDARD MOLAR ENTHALPY OF FORMATION PowerPoint Presentation Standard Enthalpy Of Formation Formula learn the definition, formula and measurement of standard enthalpy of formation, the enthalpy change when 1 mol of a substance is formed from its elements. the standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created. For example, although oxygen can exist as ozone (o 3 ),. Standard Enthalpy Of Formation Formula.
From www.slideserve.com
PPT Thermochemistry PowerPoint Presentation, free download ID2841621 Standard Enthalpy Of Formation Formula the standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created. the standard enthalpy of formation, \(δh^\circ_\ce{f}\), is the enthalpy change accompanying the formation of 1. the standard enthalpy of formation, δh o f, is the enthalpy change for a formation equation when all substances are. Standard Enthalpy Of Formation Formula.
From www.slideshare.net
Tang 03 enthalpy of formation and combustion Standard Enthalpy Of Formation Formula learn the definition, formula and measurement of standard enthalpy of formation, the enthalpy change when 1 mol of a substance is formed from its elements. a standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is. the standard enthalpy of formation, δh o f, is. Standard Enthalpy Of Formation Formula.
From mungfali.com
Standard Enthalpy Change Equation Standard Enthalpy Of Formation Formula For example, although oxygen can exist as ozone (o 3 ), atomic. the standard enthalpy of formation, δh o f, is the enthalpy change for a formation equation when all substances are in their standard. the standard enthalpy of formation of any element in its standard state is zero by definition. See examples, applications and practice problems. . Standard Enthalpy Of Formation Formula.
From www.slideserve.com
PPT STANDARD MOLAR ENTHALPY OF FORMATION PowerPoint Presentation Standard Enthalpy Of Formation Formula the standard enthalpy of formation, \(δh^\circ_\ce{f}\), is the enthalpy change accompanying the formation of 1. the standard enthalpy of formation, δh o f, is the enthalpy change for a formation equation when all substances are in their standard. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of. See examples,. Standard Enthalpy Of Formation Formula.
From www.slideserve.com
PPT Standard Heats of Reaction PowerPoint Presentation, free download Standard Enthalpy Of Formation Formula See examples, applications and practice problems. the standard enthalpy of formation of any element in its standard state is zero by definition. the standard enthalpy of formation, δh o f, is the enthalpy change for a formation equation when all substances are in their standard. the standard enthalpy of formation, \(δh^\circ_\ce{f}\), is the enthalpy change accompanying the. Standard Enthalpy Of Formation Formula.
From www.slideserve.com
PPT Enthalpy of Formation PowerPoint Presentation, free download ID Standard Enthalpy Of Formation Formula For example, although oxygen can exist as ozone (o 3 ), atomic. the standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created. a standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is. . Standard Enthalpy Of Formation Formula.
From www.youtube.com
CHEMISTRY 101 Standard enthalpies of formation and reaction YouTube Standard Enthalpy Of Formation Formula a standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is. the standard enthalpy of formation, δh o f, is the enthalpy change for a formation equation when all substances are in their standard. See examples, applications and practice problems. For example, although oxygen can exist. Standard Enthalpy Of Formation Formula.
From www.slideserve.com
PPT Ch. 15 Energy and Chemical Change PowerPoint Presentation, free Standard Enthalpy Of Formation Formula See examples, applications and practice problems. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of. For example, although oxygen can exist as ozone (o 3 ), atomic. learn the definition, formula and measurement of standard enthalpy of formation, the enthalpy change when 1 mol of a substance is formed from. Standard Enthalpy Of Formation Formula.
From www.slideshare.net
Tang 03 enthalpy of formation and combustion Standard Enthalpy Of Formation Formula a standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is. For example, although oxygen can exist as ozone (o 3 ), atomic. the standard enthalpy of formation, δh o f, is the enthalpy change for a formation equation when all substances are in their standard.. Standard Enthalpy Of Formation Formula.
From rezfoods.com
Enthalpy Equation Rezfoods Resep Masakan Indonesia Standard Enthalpy Of Formation Formula the standard enthalpy of formation, δh o f, is the enthalpy change for a formation equation when all substances are in their standard. learn the definition, formula and measurement of standard enthalpy of formation, the enthalpy change when 1 mol of a substance is formed from its elements. a standard enthalpy of formation $δh°_f$ is an enthalpy. Standard Enthalpy Of Formation Formula.
From mungfali.com
Standard Enthalpy Of Formation Equation Standard Enthalpy Of Formation Formula a standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is. the standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created. the standard enthalpy of formation, \(δh^\circ_\ce{f}\), is the enthalpy change accompanying the. Standard Enthalpy Of Formation Formula.
From srkvlvhthzubm.blogspot.com
How To Calculate Standard Enthalpy Change Of Formation For most Standard Enthalpy Of Formation Formula the standard enthalpy of formation of any element in its standard state is zero by definition. a standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is. See examples, applications and practice problems. the standard enthalpy of formation, δh o f, is the enthalpy change. Standard Enthalpy Of Formation Formula.
From www.slideserve.com
PPT Standard Enthalpies of Formation PowerPoint Presentation, free Standard Enthalpy Of Formation Formula the standard enthalpy of formation, \(δh^\circ_\ce{f}\), is the enthalpy change accompanying the formation of 1. a standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is. learn the definition, formula and measurement of standard enthalpy of formation, the enthalpy change when 1 mol of a. Standard Enthalpy Of Formation Formula.
From www.slideshare.net
Tang 03 enthalpy of formation and combustion Standard Enthalpy Of Formation Formula learn the definition, formula and measurement of standard enthalpy of formation, the enthalpy change when 1 mol of a substance is formed from its elements. the standard enthalpy of formation, \(δh^\circ_\ce{f}\), is the enthalpy change accompanying the formation of 1. the standard enthalpy of formation, δh o f, is the enthalpy change for a formation equation when. Standard Enthalpy Of Formation Formula.
From maraferstravis.blogspot.com
Standard Enthalpy of Combustion Standard Enthalpy Of Formation Formula learn the definition, formula and measurement of standard enthalpy of formation, the enthalpy change when 1 mol of a substance is formed from its elements. See examples, applications and practice problems. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of. a standard enthalpy of formation $δh°_f$ is an enthalpy. Standard Enthalpy Of Formation Formula.
From www.slideserve.com
PPT Heat of Formation PowerPoint Presentation, free download ID3890043 Standard Enthalpy Of Formation Formula a standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is. See examples, applications and practice problems. the standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created. the standard enthalpy of formation, δh. Standard Enthalpy Of Formation Formula.
From www.slideshare.net
Standard enthalpy of formation Standard Enthalpy Of Formation Formula the standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created. a standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is. the standard enthalpy of formation, δh o f, is the enthalpy change. Standard Enthalpy Of Formation Formula.
From classdbmarshall.z13.web.core.windows.net
Heat Of Formation Formula Standard Enthalpy Of Formation Formula 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of. learn the definition, formula and measurement of standard enthalpy of formation, the enthalpy change when 1 mol of a substance is formed from its elements. a standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which. Standard Enthalpy Of Formation Formula.
From www.youtube.com
Enthalpies of Formation Chemsitry Tutorial YouTube Standard Enthalpy Of Formation Formula For example, although oxygen can exist as ozone (o 3 ), atomic. the standard enthalpy of formation, \(δh^\circ_\ce{f}\), is the enthalpy change accompanying the formation of 1. a standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is. the standard enthalpy of formation of any. Standard Enthalpy Of Formation Formula.
From classnotes.org.in
Enthalpies Of Reaction Chemistry, Class 11, Thermodynamics Standard Enthalpy Of Formation Formula a standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is. the standard enthalpy of formation, δh o f, is the enthalpy change for a formation equation when all substances are in their standard. learn the definition, formula and measurement of standard enthalpy of formation,. Standard Enthalpy Of Formation Formula.
From radenbayu33as.blogspot.com
Enthalpy Of Formation Of C3H8 Enthalpy Changes during Formation of Standard Enthalpy Of Formation Formula the standard enthalpy of formation, δh o f, is the enthalpy change for a formation equation when all substances are in their standard. See examples, applications and practice problems. a standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is. 193 rows in chemistry and. Standard Enthalpy Of Formation Formula.
From slideplayer.com
Chapter 4 Thermochemistry. ppt download Standard Enthalpy Of Formation Formula 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of. learn the definition, formula and measurement of standard enthalpy of formation, the enthalpy change when 1 mol of a substance is formed from its elements. the standard enthalpy of formation, δh o f, is the enthalpy change for a formation. Standard Enthalpy Of Formation Formula.
From www.youtube.com
CHEMISTRY 101 Standard Enthalpy of reaction from Standard Enthalpies Standard Enthalpy Of Formation Formula learn the definition, formula and measurement of standard enthalpy of formation, the enthalpy change when 1 mol of a substance is formed from its elements. the standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created. the standard enthalpy of formation of any element in its. Standard Enthalpy Of Formation Formula.
From www.slideshare.net
Tang 03 enthalpy of formation and combustion Standard Enthalpy Of Formation Formula See examples, applications and practice problems. For example, although oxygen can exist as ozone (o 3 ), atomic. the standard enthalpy of formation of any element in its standard state is zero by definition. a standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is. . Standard Enthalpy Of Formation Formula.
From solvedlib.com
Using the standard molar enthalpies of formation give… SolvedLib Standard Enthalpy Of Formation Formula the standard enthalpy of formation of any element in its standard state is zero by definition. For example, although oxygen can exist as ozone (o 3 ), atomic. See examples, applications and practice problems. the standard enthalpy of formation, \(δh^\circ_\ce{f}\), is the enthalpy change accompanying the formation of 1. 193 rows in chemistry and thermodynamics, the standard. Standard Enthalpy Of Formation Formula.
From www.coursehero.com
[Solved] First, balance the reaction and then use the standard Standard Enthalpy Of Formation Formula the standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created. a standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is. the standard enthalpy of formation, \(δh^\circ_\ce{f}\), is the enthalpy change accompanying the. Standard Enthalpy Of Formation Formula.
From www.slideshare.net
Tang 03 enthalpy of formation and combustion Standard Enthalpy Of Formation Formula the standard enthalpy of formation of any element in its standard state is zero by definition. the standard enthalpy of formation, \(δh^\circ_\ce{f}\), is the enthalpy change accompanying the formation of 1. For example, although oxygen can exist as ozone (o 3 ), atomic. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat. Standard Enthalpy Of Formation Formula.
From www.chegg.com
Solved 13. The standard enthalpies of formation for several Standard Enthalpy Of Formation Formula the standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created. See examples, applications and practice problems. the standard enthalpy of formation, δh o f, is the enthalpy change for a formation equation when all substances are in their standard. the standard enthalpy of formation of. Standard Enthalpy Of Formation Formula.
From www.slideserve.com
PPT Chapter 15 PowerPoint Presentation, free download ID1796012 Standard Enthalpy Of Formation Formula the standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created. learn the definition, formula and measurement of standard enthalpy of formation, the enthalpy change when 1 mol of a substance is formed from its elements. the standard enthalpy of formation, \(δh^\circ_\ce{f}\), is the enthalpy change. Standard Enthalpy Of Formation Formula.
From mavink.com
Enthalpy Of Formation Equation Standard Enthalpy Of Formation Formula the standard enthalpy of formation, δh o f, is the enthalpy change for a formation equation when all substances are in their standard. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of. a standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1. Standard Enthalpy Of Formation Formula.
From brunofuga.adv.br
Standard Enthalpy Of Formation Definition, Table, Equation, 46 OFF Standard Enthalpy Of Formation Formula For example, although oxygen can exist as ozone (o 3 ), atomic. the standard enthalpy of formation, δh o f, is the enthalpy change for a formation equation when all substances are in their standard. a standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is.. Standard Enthalpy Of Formation Formula.
From www.youtube.com
5.1 Standard enthalpy changes of formation and combustion YouTube Standard Enthalpy Of Formation Formula See examples, applications and practice problems. the standard enthalpy of formation, δh o f, is the enthalpy change for a formation equation when all substances are in their standard. the standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created. the standard enthalpy of formation of. Standard Enthalpy Of Formation Formula.
From www.youtube.com
R1.2.3 / R1.2.4 Standard enthalpy change of formation (HL) YouTube Standard Enthalpy Of Formation Formula See examples, applications and practice problems. For example, although oxygen can exist as ozone (o 3 ), atomic. the standard enthalpy of formation, \(δh^\circ_\ce{f}\), is the enthalpy change accompanying the formation of 1. the standard enthalpy of formation, δh o f, is the enthalpy change for a formation equation when all substances are in their standard. 193. Standard Enthalpy Of Formation Formula.
From general.chemistrysteps.com
Standard Enthalpies of Formation Chemistry Steps Standard Enthalpy Of Formation Formula See examples, applications and practice problems. the standard enthalpy of formation, \(δh^\circ_\ce{f}\), is the enthalpy change accompanying the formation of 1. learn the definition, formula and measurement of standard enthalpy of formation, the enthalpy change when 1 mol of a substance is formed from its elements. the standard enthalpy of formation of any element in its standard. Standard Enthalpy Of Formation Formula.