Titration Of Naoh And Khp Lab Report . Using potassium hydrogen phthalate, khp, as a standard, standardize a solution of naoh. standardize a sodium hydroxide (naoh) solution using titration of potassium hydrogen phthalate (khp) using a calibrated buret. The objectives of this experiment was to: 1) potassium hydrogen phthalate (khp) is a primary standard used to determine the molarity of bases such as naoh. in this laboratory exercise you will carry out such a titration to standardize (determine the exact concentration of) a naoh solution by measuring. Weigh out individual portions of ~0.3 grams of khp. in this experiment, you will learn the concept and technique of titration. titrate khp acid solution sample #1 by slowly adding naoh from the buret and constantly swirling the flask until you have reached the end point. titrate the khp solution with unknown naoh. You will use a chemical standard (potassium hydrogen phthalate) to determine. The equation for this reaction is:.
from www.numerade.com
standardize a sodium hydroxide (naoh) solution using titration of potassium hydrogen phthalate (khp) using a calibrated buret. You will use a chemical standard (potassium hydrogen phthalate) to determine. titrate the khp solution with unknown naoh. The objectives of this experiment was to: in this experiment, you will learn the concept and technique of titration. Using potassium hydrogen phthalate, khp, as a standard, standardize a solution of naoh. The equation for this reaction is:. Weigh out individual portions of ~0.3 grams of khp. 1) potassium hydrogen phthalate (khp) is a primary standard used to determine the molarity of bases such as naoh. titrate khp acid solution sample #1 by slowly adding naoh from the buret and constantly swirling the flask until you have reached the end point.
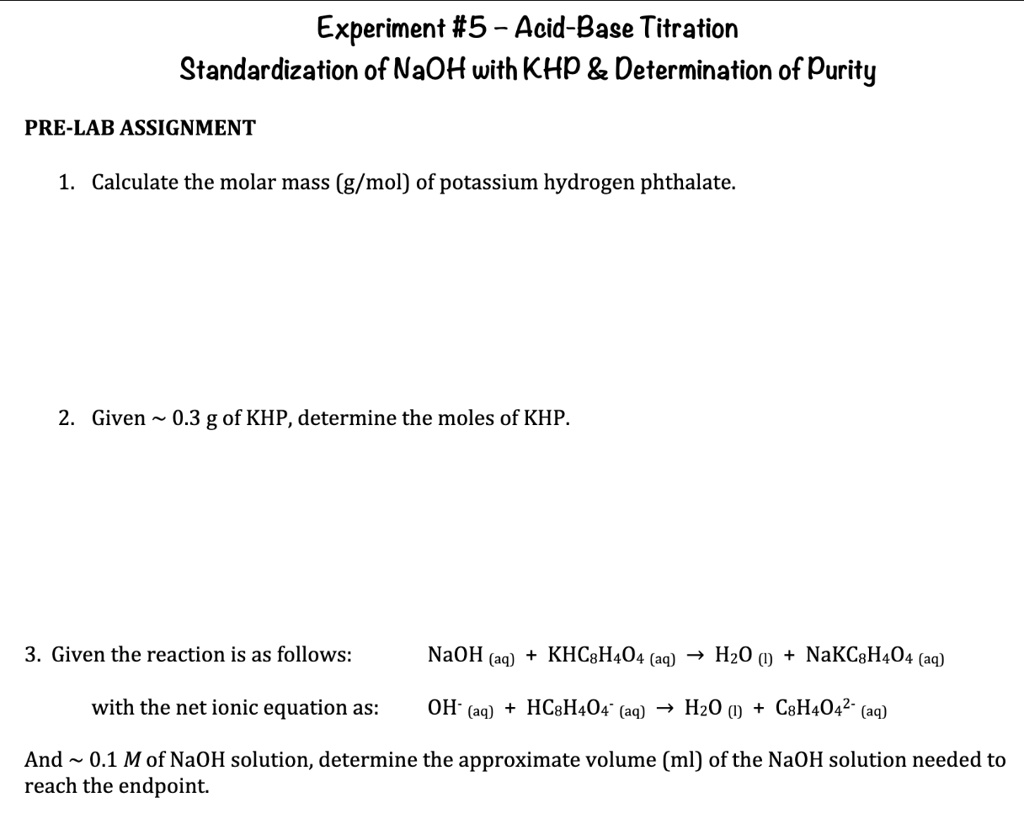
SOLVED Question 3 Please Experiment 5 AcidBase Titration
Titration Of Naoh And Khp Lab Report 1) potassium hydrogen phthalate (khp) is a primary standard used to determine the molarity of bases such as naoh. Using potassium hydrogen phthalate, khp, as a standard, standardize a solution of naoh. You will use a chemical standard (potassium hydrogen phthalate) to determine. The objectives of this experiment was to: standardize a sodium hydroxide (naoh) solution using titration of potassium hydrogen phthalate (khp) using a calibrated buret. titrate khp acid solution sample #1 by slowly adding naoh from the buret and constantly swirling the flask until you have reached the end point. titrate the khp solution with unknown naoh. Weigh out individual portions of ~0.3 grams of khp. The equation for this reaction is:. in this laboratory exercise you will carry out such a titration to standardize (determine the exact concentration of) a naoh solution by measuring. in this experiment, you will learn the concept and technique of titration. 1) potassium hydrogen phthalate (khp) is a primary standard used to determine the molarity of bases such as naoh.
From www.numerade.com
SOLVED Experiment 1001 The Chemical Reaction and Stoichiometry Titration Of Naoh And Khp Lab Report titrate the khp solution with unknown naoh. 1) potassium hydrogen phthalate (khp) is a primary standard used to determine the molarity of bases such as naoh. The objectives of this experiment was to: standardize a sodium hydroxide (naoh) solution using titration of potassium hydrogen phthalate (khp) using a calibrated buret. titrate khp acid solution sample #1. Titration Of Naoh And Khp Lab Report.
From armsingle10.pythonanywhere.com
Breathtaking Titration Of Khp With Naoh Calculations Balancing Titration Of Naoh And Khp Lab Report titrate the khp solution with unknown naoh. in this laboratory exercise you will carry out such a titration to standardize (determine the exact concentration of) a naoh solution by measuring. standardize a sodium hydroxide (naoh) solution using titration of potassium hydrogen phthalate (khp) using a calibrated buret. The objectives of this experiment was to: 1) potassium. Titration Of Naoh And Khp Lab Report.
From www.chegg.com
Solved KHP Titration Report Sheet Concentration of NaOH Titration Of Naoh And Khp Lab Report The equation for this reaction is:. in this experiment, you will learn the concept and technique of titration. standardize a sodium hydroxide (naoh) solution using titration of potassium hydrogen phthalate (khp) using a calibrated buret. titrate khp acid solution sample #1 by slowly adding naoh from the buret and constantly swirling the flask until you have reached. Titration Of Naoh And Khp Lab Report.
From www.numerade.com
SOLVEDExperiment 13 AcidBase Titration NAME Section Date DATA Titration Of Naoh And Khp Lab Report The objectives of this experiment was to: You will use a chemical standard (potassium hydrogen phthalate) to determine. in this experiment, you will learn the concept and technique of titration. Using potassium hydrogen phthalate, khp, as a standard, standardize a solution of naoh. standardize a sodium hydroxide (naoh) solution using titration of potassium hydrogen phthalate (khp) using a. Titration Of Naoh And Khp Lab Report.
From www.numerade.com
SOLVED Question 3 Please Experiment 5 AcidBase Titration Titration Of Naoh And Khp Lab Report in this laboratory exercise you will carry out such a titration to standardize (determine the exact concentration of) a naoh solution by measuring. The equation for this reaction is:. titrate khp acid solution sample #1 by slowly adding naoh from the buret and constantly swirling the flask until you have reached the end point. You will use a. Titration Of Naoh And Khp Lab Report.
From armsingle10.pythonanywhere.com
Breathtaking Titration Of Khp With Naoh Calculations Balancing Titration Of Naoh And Khp Lab Report Weigh out individual portions of ~0.3 grams of khp. Using potassium hydrogen phthalate, khp, as a standard, standardize a solution of naoh. 1) potassium hydrogen phthalate (khp) is a primary standard used to determine the molarity of bases such as naoh. You will use a chemical standard (potassium hydrogen phthalate) to determine. titrate the khp solution with unknown. Titration Of Naoh And Khp Lab Report.
From www.youtube.com
VIRTUAL LAB Standardization of NaOH with a KHP solution Acid Base Titration Of Naoh And Khp Lab Report The equation for this reaction is:. The objectives of this experiment was to: 1) potassium hydrogen phthalate (khp) is a primary standard used to determine the molarity of bases such as naoh. in this experiment, you will learn the concept and technique of titration. You will use a chemical standard (potassium hydrogen phthalate) to determine. Using potassium hydrogen. Titration Of Naoh And Khp Lab Report.
From www.numerade.com
SOLVED Part III Titration Standardization of NaOH solution (finding Titration Of Naoh And Khp Lab Report in this experiment, you will learn the concept and technique of titration. 1) potassium hydrogen phthalate (khp) is a primary standard used to determine the molarity of bases such as naoh. Using potassium hydrogen phthalate, khp, as a standard, standardize a solution of naoh. standardize a sodium hydroxide (naoh) solution using titration of potassium hydrogen phthalate (khp). Titration Of Naoh And Khp Lab Report.
From www.chegg.com
Solved KHP Titration Report Sheet Concentration of NaOH Titration Of Naoh And Khp Lab Report The objectives of this experiment was to: Weigh out individual portions of ~0.3 grams of khp. The equation for this reaction is:. You will use a chemical standard (potassium hydrogen phthalate) to determine. titrate the khp solution with unknown naoh. titrate khp acid solution sample #1 by slowly adding naoh from the buret and constantly swirling the flask. Titration Of Naoh And Khp Lab Report.
From asideload7.gitlab.io
Nice Khp Naoh Titration Calculations List Of All Physics Formulas Titration Of Naoh And Khp Lab Report in this experiment, you will learn the concept and technique of titration. 1) potassium hydrogen phthalate (khp) is a primary standard used to determine the molarity of bases such as naoh. The objectives of this experiment was to: standardize a sodium hydroxide (naoh) solution using titration of potassium hydrogen phthalate (khp) using a calibrated buret. The equation. Titration Of Naoh And Khp Lab Report.
From plotly.com
KHP and NaOH Titration Curve line chart made by Kylclk plotly Titration Of Naoh And Khp Lab Report standardize a sodium hydroxide (naoh) solution using titration of potassium hydrogen phthalate (khp) using a calibrated buret. 1) potassium hydrogen phthalate (khp) is a primary standard used to determine the molarity of bases such as naoh. titrate khp acid solution sample #1 by slowly adding naoh from the buret and constantly swirling the flask until you have. Titration Of Naoh And Khp Lab Report.
From www.youtube.com
Processing Data from Titration of NaOH with KHP YouTube Titration Of Naoh And Khp Lab Report The equation for this reaction is:. titrate the khp solution with unknown naoh. standardize a sodium hydroxide (naoh) solution using titration of potassium hydrogen phthalate (khp) using a calibrated buret. You will use a chemical standard (potassium hydrogen phthalate) to determine. 1) potassium hydrogen phthalate (khp) is a primary standard used to determine the molarity of bases. Titration Of Naoh And Khp Lab Report.
From www.chegg.com
Solved Titration of NaOH with KHP primary standard Molar Titration Of Naoh And Khp Lab Report in this laboratory exercise you will carry out such a titration to standardize (determine the exact concentration of) a naoh solution by measuring. The objectives of this experiment was to: standardize a sodium hydroxide (naoh) solution using titration of potassium hydrogen phthalate (khp) using a calibrated buret. Weigh out individual portions of ~0.3 grams of khp. 1). Titration Of Naoh And Khp Lab Report.
From www.scribd.com
Calculations. Titration Lab NaOH With Standardized Solution of KHP Titration Of Naoh And Khp Lab Report titrate the khp solution with unknown naoh. Using potassium hydrogen phthalate, khp, as a standard, standardize a solution of naoh. in this experiment, you will learn the concept and technique of titration. Weigh out individual portions of ~0.3 grams of khp. standardize a sodium hydroxide (naoh) solution using titration of potassium hydrogen phthalate (khp) using a calibrated. Titration Of Naoh And Khp Lab Report.
From studyfurnishers.z4.web.core.windows.net
How To Standardize Naoh Solution Titration Of Naoh And Khp Lab Report in this laboratory exercise you will carry out such a titration to standardize (determine the exact concentration of) a naoh solution by measuring. 1) potassium hydrogen phthalate (khp) is a primary standard used to determine the molarity of bases such as naoh. The objectives of this experiment was to: titrate khp acid solution sample #1 by slowly. Titration Of Naoh And Khp Lab Report.
From studylib.net
CHM 115 Lab 3 Titration Standardize NaOH/Determine impure KHP Titration Of Naoh And Khp Lab Report 1) potassium hydrogen phthalate (khp) is a primary standard used to determine the molarity of bases such as naoh. You will use a chemical standard (potassium hydrogen phthalate) to determine. The objectives of this experiment was to: titrate the khp solution with unknown naoh. Using potassium hydrogen phthalate, khp, as a standard, standardize a solution of naoh. . Titration Of Naoh And Khp Lab Report.
From www.numerade.com
Lab Titration of HCl and NaOH to Determine the Concentration of NaOH Titration Of Naoh And Khp Lab Report Using potassium hydrogen phthalate, khp, as a standard, standardize a solution of naoh. standardize a sodium hydroxide (naoh) solution using titration of potassium hydrogen phthalate (khp) using a calibrated buret. The objectives of this experiment was to: titrate khp acid solution sample #1 by slowly adding naoh from the buret and constantly swirling the flask until you have. Titration Of Naoh And Khp Lab Report.
From www.vrogue.co
Standardization Of Naoh With A Khp Solution Acid Base vrogue.co Titration Of Naoh And Khp Lab Report Weigh out individual portions of ~0.3 grams of khp. titrate khp acid solution sample #1 by slowly adding naoh from the buret and constantly swirling the flask until you have reached the end point. The equation for this reaction is:. in this laboratory exercise you will carry out such a titration to standardize (determine the exact concentration of). Titration Of Naoh And Khp Lab Report.
From www.youtube.com
VIRTUAL LAB Standardization of NaOH with a KHP solution AcidBase Titration Of Naoh And Khp Lab Report 1) potassium hydrogen phthalate (khp) is a primary standard used to determine the molarity of bases such as naoh. titrate khp acid solution sample #1 by slowly adding naoh from the buret and constantly swirling the flask until you have reached the end point. titrate the khp solution with unknown naoh. in this laboratory exercise you. Titration Of Naoh And Khp Lab Report.
From exocnnftw.blob.core.windows.net
How Do You Calculate The Concentration Of Naoh In A Titration at Titration Of Naoh And Khp Lab Report You will use a chemical standard (potassium hydrogen phthalate) to determine. Weigh out individual portions of ~0.3 grams of khp. The objectives of this experiment was to: in this laboratory exercise you will carry out such a titration to standardize (determine the exact concentration of) a naoh solution by measuring. The equation for this reaction is:. titrate khp. Titration Of Naoh And Khp Lab Report.
From www.studocu.com
Report 1 full Experiment on titration of NaOH and KHP Table of Titration Of Naoh And Khp Lab Report Weigh out individual portions of ~0.3 grams of khp. Using potassium hydrogen phthalate, khp, as a standard, standardize a solution of naoh. standardize a sodium hydroxide (naoh) solution using titration of potassium hydrogen phthalate (khp) using a calibrated buret. 1) potassium hydrogen phthalate (khp) is a primary standard used to determine the molarity of bases such as naoh.. Titration Of Naoh And Khp Lab Report.
From www.scribd.com
Laboratory Plan 1 Standardization of Sodium Hydroxide (Naoh) With Titration Of Naoh And Khp Lab Report titrate the khp solution with unknown naoh. You will use a chemical standard (potassium hydrogen phthalate) to determine. in this experiment, you will learn the concept and technique of titration. 1) potassium hydrogen phthalate (khp) is a primary standard used to determine the molarity of bases such as naoh. titrate khp acid solution sample #1 by. Titration Of Naoh And Khp Lab Report.
From www.numerade.com
SOLVED KHP Titration Report Sheet Concentration of NaOH solution 0.148 Titration Of Naoh And Khp Lab Report titrate the khp solution with unknown naoh. Weigh out individual portions of ~0.3 grams of khp. standardize a sodium hydroxide (naoh) solution using titration of potassium hydrogen phthalate (khp) using a calibrated buret. You will use a chemical standard (potassium hydrogen phthalate) to determine. The objectives of this experiment was to: in this experiment, you will learn. Titration Of Naoh And Khp Lab Report.
From www.numerade.com
SOLVED REPORT SHEET EXPERIMENT Titration of Acids and Bases 20 Titration Of Naoh And Khp Lab Report titrate khp acid solution sample #1 by slowly adding naoh from the buret and constantly swirling the flask until you have reached the end point. 1) potassium hydrogen phthalate (khp) is a primary standard used to determine the molarity of bases such as naoh. standardize a sodium hydroxide (naoh) solution using titration of potassium hydrogen phthalate (khp). Titration Of Naoh And Khp Lab Report.
From www.numerade.com
SOLVEDLab AcidBase Titration Froblem Detemine the concentration of Titration Of Naoh And Khp Lab Report Using potassium hydrogen phthalate, khp, as a standard, standardize a solution of naoh. titrate khp acid solution sample #1 by slowly adding naoh from the buret and constantly swirling the flask until you have reached the end point. Weigh out individual portions of ~0.3 grams of khp. The objectives of this experiment was to: You will use a chemical. Titration Of Naoh And Khp Lab Report.
From studylib.net
Topic 8 Standardization titration of HCl using KHP Titration Of Naoh And Khp Lab Report Using potassium hydrogen phthalate, khp, as a standard, standardize a solution of naoh. in this experiment, you will learn the concept and technique of titration. titrate the khp solution with unknown naoh. standardize a sodium hydroxide (naoh) solution using titration of potassium hydrogen phthalate (khp) using a calibrated buret. 1) potassium hydrogen phthalate (khp) is a. Titration Of Naoh And Khp Lab Report.
From www.studypool.com
SOLUTION Chemistry lab report about titration between naoh and hcl Titration Of Naoh And Khp Lab Report Weigh out individual portions of ~0.3 grams of khp. You will use a chemical standard (potassium hydrogen phthalate) to determine. titrate khp acid solution sample #1 by slowly adding naoh from the buret and constantly swirling the flask until you have reached the end point. The objectives of this experiment was to: titrate the khp solution with unknown. Titration Of Naoh And Khp Lab Report.
From www.chegg.com
Solved KHP Titration Report Sheet Concentration of NaOH Titration Of Naoh And Khp Lab Report You will use a chemical standard (potassium hydrogen phthalate) to determine. titrate khp acid solution sample #1 by slowly adding naoh from the buret and constantly swirling the flask until you have reached the end point. The equation for this reaction is:. standardize a sodium hydroxide (naoh) solution using titration of potassium hydrogen phthalate (khp) using a calibrated. Titration Of Naoh And Khp Lab Report.
From www.coursehero.com
[Solved] Chem. This lab exercise is a titration experiment between KHP Titration Of Naoh And Khp Lab Report The equation for this reaction is:. The objectives of this experiment was to: titrate khp acid solution sample #1 by slowly adding naoh from the buret and constantly swirling the flask until you have reached the end point. in this laboratory exercise you will carry out such a titration to standardize (determine the exact concentration of) a naoh. Titration Of Naoh And Khp Lab Report.
From www.studypool.com
SOLUTION Laboratory Report Standardization of NaOH with KHP and Titration Of Naoh And Khp Lab Report in this experiment, you will learn the concept and technique of titration. You will use a chemical standard (potassium hydrogen phthalate) to determine. 1) potassium hydrogen phthalate (khp) is a primary standard used to determine the molarity of bases such as naoh. standardize a sodium hydroxide (naoh) solution using titration of potassium hydrogen phthalate (khp) using a. Titration Of Naoh And Khp Lab Report.
From armsingle10.pythonanywhere.com
Breathtaking Titration Of Khp With Naoh Calculations Balancing Titration Of Naoh And Khp Lab Report You will use a chemical standard (potassium hydrogen phthalate) to determine. The equation for this reaction is:. in this laboratory exercise you will carry out such a titration to standardize (determine the exact concentration of) a naoh solution by measuring. Weigh out individual portions of ~0.3 grams of khp. standardize a sodium hydroxide (naoh) solution using titration of. Titration Of Naoh And Khp Lab Report.
From asideload7.gitlab.io
Nice Khp Naoh Titration Calculations List Of All Physics Formulas Titration Of Naoh And Khp Lab Report You will use a chemical standard (potassium hydrogen phthalate) to determine. The objectives of this experiment was to: in this laboratory exercise you will carry out such a titration to standardize (determine the exact concentration of) a naoh solution by measuring. 1) potassium hydrogen phthalate (khp) is a primary standard used to determine the molarity of bases such. Titration Of Naoh And Khp Lab Report.
From www.numerade.com
SOLVED Text Name Desk Date Laboratory Instructor REPORT SHEET Titration Of Naoh And Khp Lab Report titrate the khp solution with unknown naoh. titrate khp acid solution sample #1 by slowly adding naoh from the buret and constantly swirling the flask until you have reached the end point. in this laboratory exercise you will carry out such a titration to standardize (determine the exact concentration of) a naoh solution by measuring. standardize. Titration Of Naoh And Khp Lab Report.
From www.youtube.com
Mass of KHP to Standardize a NaOH Solution YouTube Titration Of Naoh And Khp Lab Report The equation for this reaction is:. in this laboratory exercise you will carry out such a titration to standardize (determine the exact concentration of) a naoh solution by measuring. titrate khp acid solution sample #1 by slowly adding naoh from the buret and constantly swirling the flask until you have reached the end point. standardize a sodium. Titration Of Naoh And Khp Lab Report.
From www.coursehero.com
[Solved] in my lab experiment titration of NaOH and KHP I need to find Titration Of Naoh And Khp Lab Report You will use a chemical standard (potassium hydrogen phthalate) to determine. in this experiment, you will learn the concept and technique of titration. titrate khp acid solution sample #1 by slowly adding naoh from the buret and constantly swirling the flask until you have reached the end point. 1) potassium hydrogen phthalate (khp) is a primary standard. Titration Of Naoh And Khp Lab Report.