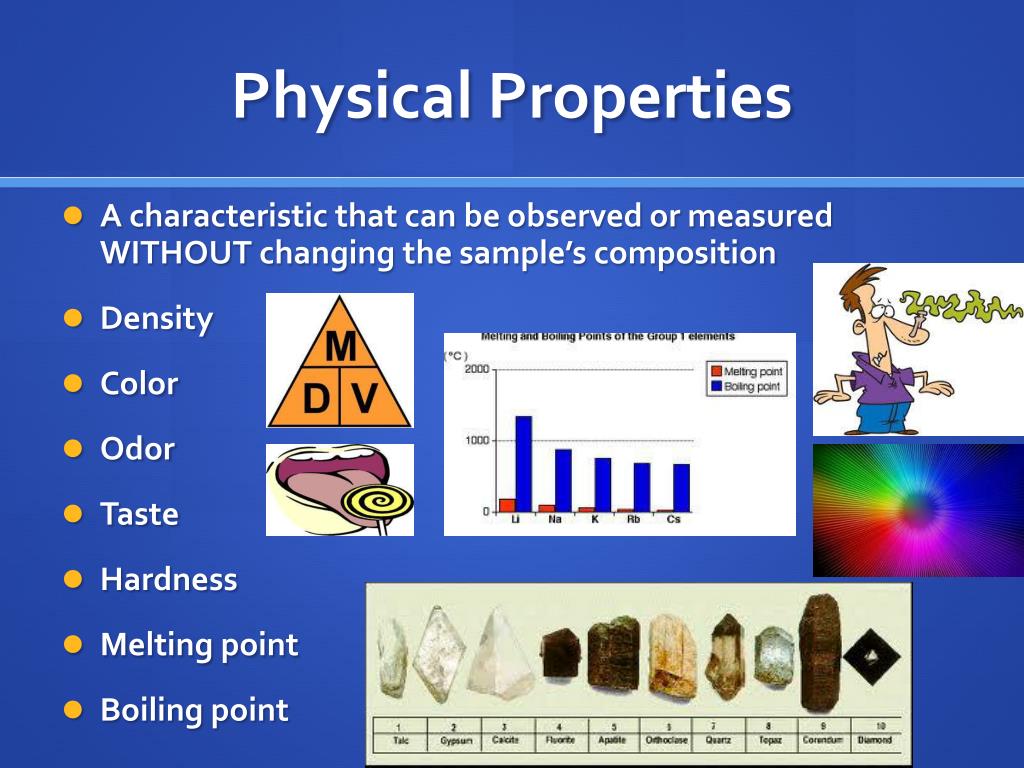
What Are The Observable Properties Of Salt . The properties of a salt largely depend on the type of salt that it is. the reactants are composed of the salt and the water and the products side is composed of the conjugate base (from the acid of the. The reaction of a salt with water to. Acids conduct an electric current. properties of salts in chemistry. Sulfuric acid and sodium chloride react to form sodium sulphate and hydrogen chloride. Salt is essential to the health of both people and animals. Bases conduct an electric current. identifying different types of salts is. chemical properties of sodium chloride. salts that contain small, highly charged metal ions produce acidic solutions in water. But, salts display characteristics relating to.
from www.slideserve.com
The reaction of a salt with water to. Bases conduct an electric current. chemical properties of sodium chloride. Acids conduct an electric current. Salt is essential to the health of both people and animals. identifying different types of salts is. the reactants are composed of the salt and the water and the products side is composed of the conjugate base (from the acid of the. But, salts display characteristics relating to. The properties of a salt largely depend on the type of salt that it is. Sulfuric acid and sodium chloride react to form sodium sulphate and hydrogen chloride.
PPT MatterProperties and Changes PowerPoint Presentation, free
What Are The Observable Properties Of Salt Sulfuric acid and sodium chloride react to form sodium sulphate and hydrogen chloride. identifying different types of salts is. chemical properties of sodium chloride. But, salts display characteristics relating to. The properties of a salt largely depend on the type of salt that it is. Salt is essential to the health of both people and animals. Acids conduct an electric current. Sulfuric acid and sodium chloride react to form sodium sulphate and hydrogen chloride. properties of salts in chemistry. salts that contain small, highly charged metal ions produce acidic solutions in water. the reactants are composed of the salt and the water and the products side is composed of the conjugate base (from the acid of the. Bases conduct an electric current. The reaction of a salt with water to.
From www.slideserve.com
PPT MatterProperties and Changes PowerPoint Presentation, free What Are The Observable Properties Of Salt The reaction of a salt with water to. But, salts display characteristics relating to. The properties of a salt largely depend on the type of salt that it is. salts that contain small, highly charged metal ions produce acidic solutions in water. Bases conduct an electric current. chemical properties of sodium chloride. Acids conduct an electric current. . What Are The Observable Properties Of Salt.
From www.careerpower.in
Salt Chemistry Definition, Types, Properties, Hydrolysis of Salt What Are The Observable Properties Of Salt properties of salts in chemistry. But, salts display characteristics relating to. identifying different types of salts is. The properties of a salt largely depend on the type of salt that it is. chemical properties of sodium chloride. Salt is essential to the health of both people and animals. salts that contain small, highly charged metal ions. What Are The Observable Properties Of Salt.
From www.slideserve.com
PPT Ionic Compounds PowerPoint Presentation, free download ID2422375 What Are The Observable Properties Of Salt The properties of a salt largely depend on the type of salt that it is. chemical properties of sodium chloride. The reaction of a salt with water to. identifying different types of salts is. But, salts display characteristics relating to. Bases conduct an electric current. properties of salts in chemistry. Acids conduct an electric current. the. What Are The Observable Properties Of Salt.
From www.slideserve.com
PPT Acids and Bases PowerPoint Presentation, free download ID2793569 What Are The Observable Properties Of Salt salts that contain small, highly charged metal ions produce acidic solutions in water. The reaction of a salt with water to. chemical properties of sodium chloride. Sulfuric acid and sodium chloride react to form sodium sulphate and hydrogen chloride. properties of salts in chemistry. But, salts display characteristics relating to. identifying different types of salts is.. What Are The Observable Properties Of Salt.
From www.slideshare.net
types and properties of salts What Are The Observable Properties Of Salt the reactants are composed of the salt and the water and the products side is composed of the conjugate base (from the acid of the. properties of salts in chemistry. Bases conduct an electric current. Acids conduct an electric current. But, salts display characteristics relating to. The reaction of a salt with water to. Salt is essential to. What Are The Observable Properties Of Salt.
From chemisfast.blogspot.com
Double saltsdefinitionexamples and properties in coordination What Are The Observable Properties Of Salt Bases conduct an electric current. identifying different types of salts is. the reactants are composed of the salt and the water and the products side is composed of the conjugate base (from the acid of the. salts that contain small, highly charged metal ions produce acidic solutions in water. properties of salts in chemistry. The reaction. What Are The Observable Properties Of Salt.
From definitionklw.blogspot.com
Definition Of Salt In Chemistry DEFINITION KLW What Are The Observable Properties Of Salt salts that contain small, highly charged metal ions produce acidic solutions in water. the reactants are composed of the salt and the water and the products side is composed of the conjugate base (from the acid of the. The properties of a salt largely depend on the type of salt that it is. Acids conduct an electric current.. What Are The Observable Properties Of Salt.
From www.researchgate.net
List of salts and their properties Download Scientific Diagram What Are The Observable Properties Of Salt Salt is essential to the health of both people and animals. properties of salts in chemistry. salts that contain small, highly charged metal ions produce acidic solutions in water. Acids conduct an electric current. Sulfuric acid and sodium chloride react to form sodium sulphate and hydrogen chloride. The reaction of a salt with water to. the reactants. What Are The Observable Properties Of Salt.
From www.sliderbase.com
AcidBase Properties of Salts Presentation Chemistry What Are The Observable Properties Of Salt properties of salts in chemistry. Acids conduct an electric current. salts that contain small, highly charged metal ions produce acidic solutions in water. identifying different types of salts is. The reaction of a salt with water to. Salt is essential to the health of both people and animals. The properties of a salt largely depend on the. What Are The Observable Properties Of Salt.
From aminghori.blogspot.com
Lesson Plan of Properties and uses of Salt General Science Grade VIII What Are The Observable Properties Of Salt salts that contain small, highly charged metal ions produce acidic solutions in water. But, salts display characteristics relating to. identifying different types of salts is. The properties of a salt largely depend on the type of salt that it is. Salt is essential to the health of both people and animals. Bases conduct an electric current. Acids conduct. What Are The Observable Properties Of Salt.
From www.slideserve.com
PPT Chapter 4 PowerPoint Presentation, free download ID1980306 What Are The Observable Properties Of Salt But, salts display characteristics relating to. The reaction of a salt with water to. The properties of a salt largely depend on the type of salt that it is. salts that contain small, highly charged metal ions produce acidic solutions in water. Bases conduct an electric current. Acids conduct an electric current. Sulfuric acid and sodium chloride react to. What Are The Observable Properties Of Salt.
From www.youtube.com
Properties of salts Chemical And Physical Properties of salts What Are The Observable Properties Of Salt identifying different types of salts is. salts that contain small, highly charged metal ions produce acidic solutions in water. The properties of a salt largely depend on the type of salt that it is. Acids conduct an electric current. the reactants are composed of the salt and the water and the products side is composed of the. What Are The Observable Properties Of Salt.
From curiosityguide.org
Salts. Most Common Salts and Their Characteristics What Are The Observable Properties Of Salt Salt is essential to the health of both people and animals. properties of salts in chemistry. The reaction of a salt with water to. Bases conduct an electric current. identifying different types of salts is. salts that contain small, highly charged metal ions produce acidic solutions in water. But, salts display characteristics relating to. Acids conduct an. What Are The Observable Properties Of Salt.
From www.youtube.com
SaltsTypes of saltsAcids,Bases and SaltsProperties of saltsClass 10 What Are The Observable Properties Of Salt salts that contain small, highly charged metal ions produce acidic solutions in water. chemical properties of sodium chloride. properties of salts in chemistry. Bases conduct an electric current. Sulfuric acid and sodium chloride react to form sodium sulphate and hydrogen chloride. But, salts display characteristics relating to. Salt is essential to the health of both people and. What Are The Observable Properties Of Salt.
From pediaa.com
Difference Between Double Salt and Complex Salt Definition What Are The Observable Properties Of Salt Acids conduct an electric current. identifying different types of salts is. Sulfuric acid and sodium chloride react to form sodium sulphate and hydrogen chloride. The properties of a salt largely depend on the type of salt that it is. But, salts display characteristics relating to. properties of salts in chemistry. the reactants are composed of the salt. What Are The Observable Properties Of Salt.
From mydigitalkemistry.com
Salt Definition, Examples, Properties and Uses Chemistry Best What Are The Observable Properties Of Salt Sulfuric acid and sodium chloride react to form sodium sulphate and hydrogen chloride. Acids conduct an electric current. Salt is essential to the health of both people and animals. The reaction of a salt with water to. properties of salts in chemistry. Bases conduct an electric current. The properties of a salt largely depend on the type of salt. What Are The Observable Properties Of Salt.
From www.slideserve.com
PPT AcidBase Properties of Salt Solutions PowerPoint Presentation What Are The Observable Properties Of Salt Acids conduct an electric current. Bases conduct an electric current. The properties of a salt largely depend on the type of salt that it is. But, salts display characteristics relating to. Salt is essential to the health of both people and animals. Sulfuric acid and sodium chloride react to form sodium sulphate and hydrogen chloride. the reactants are composed. What Are The Observable Properties Of Salt.
From www.slideshare.net
Ocean properties compilation What Are The Observable Properties Of Salt chemical properties of sodium chloride. But, salts display characteristics relating to. identifying different types of salts is. properties of salts in chemistry. the reactants are composed of the salt and the water and the products side is composed of the conjugate base (from the acid of the. Acids conduct an electric current. Salt is essential to. What Are The Observable Properties Of Salt.
From www.slideserve.com
PPT Chapter 3 Matter and Energy PowerPoint Presentation, free What Are The Observable Properties Of Salt Bases conduct an electric current. Sulfuric acid and sodium chloride react to form sodium sulphate and hydrogen chloride. properties of salts in chemistry. the reactants are composed of the salt and the water and the products side is composed of the conjugate base (from the acid of the. identifying different types of salts is. Acids conduct an. What Are The Observable Properties Of Salt.
From www.slideserve.com
PPT Chapter 5 Ionic Bonding PowerPoint Presentation, free download What Are The Observable Properties Of Salt The reaction of a salt with water to. the reactants are composed of the salt and the water and the products side is composed of the conjugate base (from the acid of the. chemical properties of sodium chloride. identifying different types of salts is. But, salts display characteristics relating to. properties of salts in chemistry. Sulfuric. What Are The Observable Properties Of Salt.
From www.teachoo.com
Salts and it's Properties (with Examples) Acids, Bases and Salt What Are The Observable Properties Of Salt But, salts display characteristics relating to. Acids conduct an electric current. salts that contain small, highly charged metal ions produce acidic solutions in water. properties of salts in chemistry. identifying different types of salts is. The properties of a salt largely depend on the type of salt that it is. Bases conduct an electric current. chemical. What Are The Observable Properties Of Salt.
From www.sliderbase.com
AcidBase Properties of Salts Presentation Chemistry What Are The Observable Properties Of Salt identifying different types of salts is. salts that contain small, highly charged metal ions produce acidic solutions in water. The properties of a salt largely depend on the type of salt that it is. the reactants are composed of the salt and the water and the products side is composed of the conjugate base (from the acid. What Are The Observable Properties Of Salt.
From www.slideserve.com
PPT Salt Water PowerPoint Presentation, free download ID7029838 What Are The Observable Properties Of Salt Acids conduct an electric current. The reaction of a salt with water to. Sulfuric acid and sodium chloride react to form sodium sulphate and hydrogen chloride. Salt is essential to the health of both people and animals. But, salts display characteristics relating to. Bases conduct an electric current. The properties of a salt largely depend on the type of salt. What Are The Observable Properties Of Salt.
From www.slideserve.com
PPT AcidBase Properties of Salt Solutions PowerPoint Presentation What Are The Observable Properties Of Salt Salt is essential to the health of both people and animals. The properties of a salt largely depend on the type of salt that it is. the reactants are composed of the salt and the water and the products side is composed of the conjugate base (from the acid of the. Acids conduct an electric current. The reaction of. What Are The Observable Properties Of Salt.
From studylib.net
Properties of table salt What Are The Observable Properties Of Salt The reaction of a salt with water to. the reactants are composed of the salt and the water and the products side is composed of the conjugate base (from the acid of the. Salt is essential to the health of both people and animals. salts that contain small, highly charged metal ions produce acidic solutions in water. . What Are The Observable Properties Of Salt.
From www.slideserve.com
PPT Chapter 4 Formation of Compounds PowerPoint Presentation, free What Are The Observable Properties Of Salt Acids conduct an electric current. chemical properties of sodium chloride. the reactants are composed of the salt and the water and the products side is composed of the conjugate base (from the acid of the. Salt is essential to the health of both people and animals. salts that contain small, highly charged metal ions produce acidic solutions. What Are The Observable Properties Of Salt.
From www.slideserve.com
PPT AcidBase Properties of Salt Solutions PowerPoint Presentation What Are The Observable Properties Of Salt The reaction of a salt with water to. The properties of a salt largely depend on the type of salt that it is. the reactants are composed of the salt and the water and the products side is composed of the conjugate base (from the acid of the. Salt is essential to the health of both people and animals.. What Are The Observable Properties Of Salt.
From studylib.net
Acid Base Properties of Salt Solutions What Are The Observable Properties Of Salt identifying different types of salts is. salts that contain small, highly charged metal ions produce acidic solutions in water. properties of salts in chemistry. The properties of a salt largely depend on the type of salt that it is. Acids conduct an electric current. Salt is essential to the health of both people and animals. But, salts. What Are The Observable Properties Of Salt.
From www.careerpower.in
Salt Chemistry Definition, Types, Properties, Hydrolysis of Salt What Are The Observable Properties Of Salt Salt is essential to the health of both people and animals. Sulfuric acid and sodium chloride react to form sodium sulphate and hydrogen chloride. Bases conduct an electric current. chemical properties of sodium chloride. the reactants are composed of the salt and the water and the products side is composed of the conjugate base (from the acid of. What Are The Observable Properties Of Salt.
From www.pinterest.jp
Salt benefits infographic Physical Properties, Home Food, Our Body What Are The Observable Properties Of Salt The properties of a salt largely depend on the type of salt that it is. salts that contain small, highly charged metal ions produce acidic solutions in water. chemical properties of sodium chloride. the reactants are composed of the salt and the water and the products side is composed of the conjugate base (from the acid of. What Are The Observable Properties Of Salt.
From www.researchgate.net
A general summary of the characteristics of saltaffected soils What Are The Observable Properties Of Salt The properties of a salt largely depend on the type of salt that it is. properties of salts in chemistry. salts that contain small, highly charged metal ions produce acidic solutions in water. the reactants are composed of the salt and the water and the products side is composed of the conjugate base (from the acid of. What Are The Observable Properties Of Salt.
From sciencenotes.org
What Is a Salt in Chemistry? Definition and Examples What Are The Observable Properties Of Salt identifying different types of salts is. The properties of a salt largely depend on the type of salt that it is. chemical properties of sodium chloride. salts that contain small, highly charged metal ions produce acidic solutions in water. Sulfuric acid and sodium chloride react to form sodium sulphate and hydrogen chloride. the reactants are composed. What Are The Observable Properties Of Salt.
From abhyasonline.in
Concept Explanation What Are The Observable Properties Of Salt Sulfuric acid and sodium chloride react to form sodium sulphate and hydrogen chloride. Bases conduct an electric current. properties of salts in chemistry. chemical properties of sodium chloride. Acids conduct an electric current. Salt is essential to the health of both people and animals. identifying different types of salts is. But, salts display characteristics relating to. . What Are The Observable Properties Of Salt.
From www.slideserve.com
PPT AcidBase Properties of Salts PowerPoint Presentation, free What Are The Observable Properties Of Salt Sulfuric acid and sodium chloride react to form sodium sulphate and hydrogen chloride. Bases conduct an electric current. But, salts display characteristics relating to. identifying different types of salts is. the reactants are composed of the salt and the water and the products side is composed of the conjugate base (from the acid of the. The properties of. What Are The Observable Properties Of Salt.
From www.slideserve.com
PPT Acids and Bases PowerPoint Presentation, free download ID2793569 What Are The Observable Properties Of Salt identifying different types of salts is. salts that contain small, highly charged metal ions produce acidic solutions in water. Salt is essential to the health of both people and animals. Bases conduct an electric current. But, salts display characteristics relating to. Acids conduct an electric current. chemical properties of sodium chloride. the reactants are composed of. What Are The Observable Properties Of Salt.