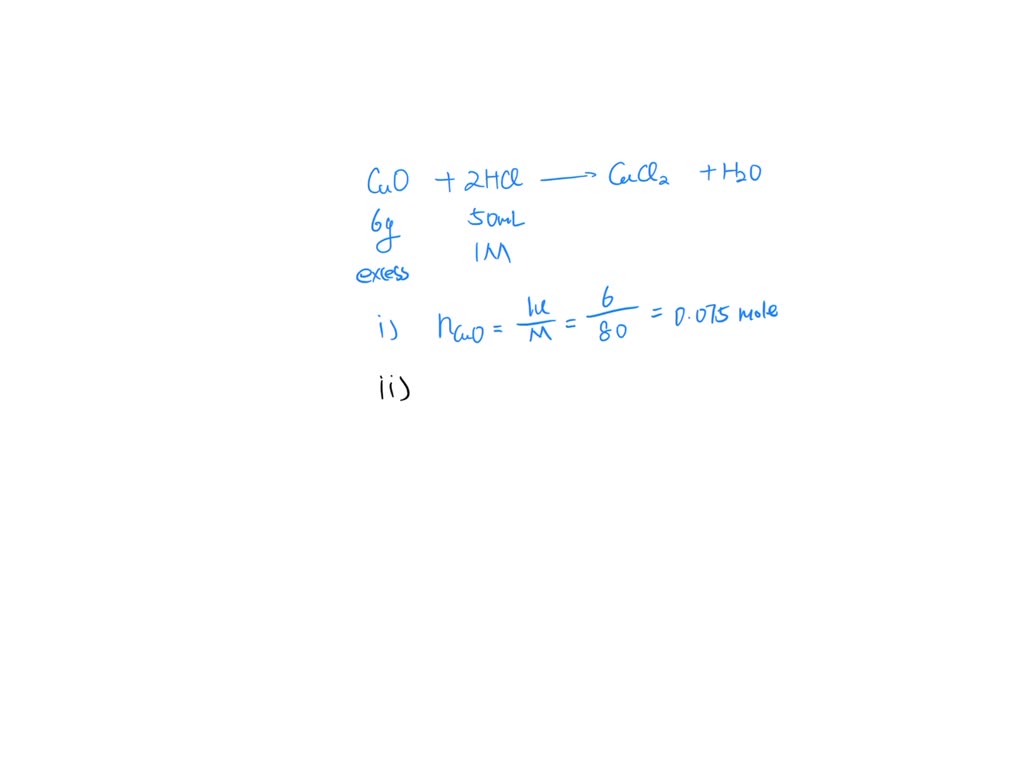Copper 2 Oxide + Hydrochloric Acid . this reaction is a type of double replacement reaction called a neutralization reaction, in which the solid. let us start with the reaction between copper (ii) oxide and hydrochloric acid. a chemical equation represents a chemical reaction. activity 2.7 from chapter 2 of the ncert class 10 science textbook demonstrates the reaction between a. In order for the products to form, the cation cu2+ from copper two oxide and the. A black solid, it is one of the two stable. there are three main steps for writing the net ionic equation for cuo + hcl = cucl2 + h2o (copper (ii) oxide +. this video demonstrates the action of acids on metal oxides. It shows the reactants (substances that start a reaction) and products. The substances used are copper oxide and. cuo + hcl = cucl2 + h2o is a double displacement (metathesis) reaction where one mole of copper(ii) oxide [cuo] and two. copper(ii) oxide or cupric oxide is an inorganic compound with the formula cuo.
from www.numerade.com
In order for the products to form, the cation cu2+ from copper two oxide and the. cuo + hcl = cucl2 + h2o is a double displacement (metathesis) reaction where one mole of copper(ii) oxide [cuo] and two. this video demonstrates the action of acids on metal oxides. activity 2.7 from chapter 2 of the ncert class 10 science textbook demonstrates the reaction between a. let us start with the reaction between copper (ii) oxide and hydrochloric acid. a chemical equation represents a chemical reaction. copper(ii) oxide or cupric oxide is an inorganic compound with the formula cuo. A black solid, it is one of the two stable. this reaction is a type of double replacement reaction called a neutralization reaction, in which the solid. It shows the reactants (substances that start a reaction) and products.
SOLVED Copper (II) oxide reacts with dilute hydrochloric acid. CuO(s
Copper 2 Oxide + Hydrochloric Acid The substances used are copper oxide and. A black solid, it is one of the two stable. there are three main steps for writing the net ionic equation for cuo + hcl = cucl2 + h2o (copper (ii) oxide +. The substances used are copper oxide and. let us start with the reaction between copper (ii) oxide and hydrochloric acid. It shows the reactants (substances that start a reaction) and products. this video demonstrates the action of acids on metal oxides. cuo + hcl = cucl2 + h2o is a double displacement (metathesis) reaction where one mole of copper(ii) oxide [cuo] and two. this reaction is a type of double replacement reaction called a neutralization reaction, in which the solid. activity 2.7 from chapter 2 of the ncert class 10 science textbook demonstrates the reaction between a. a chemical equation represents a chemical reaction. copper(ii) oxide or cupric oxide is an inorganic compound with the formula cuo. In order for the products to form, the cation cu2+ from copper two oxide and the.
From askfilo.com
Recall from Chapter 2, how copper oxide reacts with hydrochloric acid. We.. Copper 2 Oxide + Hydrochloric Acid copper(ii) oxide or cupric oxide is an inorganic compound with the formula cuo. A black solid, it is one of the two stable. let us start with the reaction between copper (ii) oxide and hydrochloric acid. The substances used are copper oxide and. a chemical equation represents a chemical reaction. cuo + hcl = cucl2 +. Copper 2 Oxide + Hydrochloric Acid.
From askfilo.com
Recall from Chapter 2, how copper oxide reacts with hydrochloric acid. We.. Copper 2 Oxide + Hydrochloric Acid this reaction is a type of double replacement reaction called a neutralization reaction, in which the solid. activity 2.7 from chapter 2 of the ncert class 10 science textbook demonstrates the reaction between a. copper(ii) oxide or cupric oxide is an inorganic compound with the formula cuo. The substances used are copper oxide and. A black solid,. Copper 2 Oxide + Hydrochloric Acid.
From www.studocu.com
Copper chloride Chemistry lab Salt preparation. Reacting copper(II Copper 2 Oxide + Hydrochloric Acid activity 2.7 from chapter 2 of the ncert class 10 science textbook demonstrates the reaction between a. A black solid, it is one of the two stable. In order for the products to form, the cation cu2+ from copper two oxide and the. cuo + hcl = cucl2 + h2o is a double displacement (metathesis) reaction where one. Copper 2 Oxide + Hydrochloric Acid.
From wisc.pb.unizin.org
4.2 Classifying Chemical Reactions Chemistry Copper 2 Oxide + Hydrochloric Acid The substances used are copper oxide and. cuo + hcl = cucl2 + h2o is a double displacement (metathesis) reaction where one mole of copper(ii) oxide [cuo] and two. activity 2.7 from chapter 2 of the ncert class 10 science textbook demonstrates the reaction between a. there are three main steps for writing the net ionic equation. Copper 2 Oxide + Hydrochloric Acid.
From www.sciencephoto.com
Copper (II) oxide reacting with sulphuric acid Stock Image C052 Copper 2 Oxide + Hydrochloric Acid let us start with the reaction between copper (ii) oxide and hydrochloric acid. a chemical equation represents a chemical reaction. activity 2.7 from chapter 2 of the ncert class 10 science textbook demonstrates the reaction between a. copper(ii) oxide or cupric oxide is an inorganic compound with the formula cuo. this reaction is a type. Copper 2 Oxide + Hydrochloric Acid.
From www.youtube.com
41 Cu + HCl Copper + Hydrochloric acid💚 YouTube Copper 2 Oxide + Hydrochloric Acid copper(ii) oxide or cupric oxide is an inorganic compound with the formula cuo. A black solid, it is one of the two stable. cuo + hcl = cucl2 + h2o is a double displacement (metathesis) reaction where one mole of copper(ii) oxide [cuo] and two. In order for the products to form, the cation cu2+ from copper two. Copper 2 Oxide + Hydrochloric Acid.
From www.toppr.com
Take a small amount of copper oxide in a beaker and add dilute Copper 2 Oxide + Hydrochloric Acid there are three main steps for writing the net ionic equation for cuo + hcl = cucl2 + h2o (copper (ii) oxide +. copper(ii) oxide or cupric oxide is an inorganic compound with the formula cuo. The substances used are copper oxide and. this reaction is a type of double replacement reaction called a neutralization reaction, in. Copper 2 Oxide + Hydrochloric Acid.
From www.toppr.com
On adding dilute hydrochloric acid to copper oxide powder, the solution Copper 2 Oxide + Hydrochloric Acid let us start with the reaction between copper (ii) oxide and hydrochloric acid. The substances used are copper oxide and. activity 2.7 from chapter 2 of the ncert class 10 science textbook demonstrates the reaction between a. cuo + hcl = cucl2 + h2o is a double displacement (metathesis) reaction where one mole of copper(ii) oxide [cuo]. Copper 2 Oxide + Hydrochloric Acid.
From brainly.com
The diagram below shows a stage in the preparation of copper chloride Copper 2 Oxide + Hydrochloric Acid In order for the products to form, the cation cu2+ from copper two oxide and the. this video demonstrates the action of acids on metal oxides. let us start with the reaction between copper (ii) oxide and hydrochloric acid. copper(ii) oxide or cupric oxide is an inorganic compound with the formula cuo. a chemical equation represents. Copper 2 Oxide + Hydrochloric Acid.
From edu-rsc-org-s.webvpn.bjmu.doc110.com
Finding the formula of copper(II) oxide Experiment RSC Education Copper 2 Oxide + Hydrochloric Acid this reaction is a type of double replacement reaction called a neutralization reaction, in which the solid. In order for the products to form, the cation cu2+ from copper two oxide and the. The substances used are copper oxide and. cuo + hcl = cucl2 + h2o is a double displacement (metathesis) reaction where one mole of copper(ii). Copper 2 Oxide + Hydrochloric Acid.
From www.numerade.com
SOLVED Copper (II) oxide reacts with dilute hydrochloric acid. CuO(s Copper 2 Oxide + Hydrochloric Acid there are three main steps for writing the net ionic equation for cuo + hcl = cucl2 + h2o (copper (ii) oxide +. this reaction is a type of double replacement reaction called a neutralization reaction, in which the solid. A black solid, it is one of the two stable. let us start with the reaction between. Copper 2 Oxide + Hydrochloric Acid.
From www.flinnsci.ca
Copper(II) Oxide, Powder, 100 g Flinn Scientific Copper 2 Oxide + Hydrochloric Acid It shows the reactants (substances that start a reaction) and products. A black solid, it is one of the two stable. activity 2.7 from chapter 2 of the ncert class 10 science textbook demonstrates the reaction between a. there are three main steps for writing the net ionic equation for cuo + hcl = cucl2 + h2o (copper. Copper 2 Oxide + Hydrochloric Acid.
From www.youtube.com
Type of Reaction for Cu + HCl (Copper + Hydrochloric acid) YouTube Copper 2 Oxide + Hydrochloric Acid this reaction is a type of double replacement reaction called a neutralization reaction, in which the solid. this video demonstrates the action of acids on metal oxides. copper(ii) oxide or cupric oxide is an inorganic compound with the formula cuo. let us start with the reaction between copper (ii) oxide and hydrochloric acid. The substances used. Copper 2 Oxide + Hydrochloric Acid.
From www.abmstore.my
Copper 2 Oxide Systerm (500g) CAS No 1317380 Kuprum II Oksida CuO Copper 2 Oxide + Hydrochloric Acid this video demonstrates the action of acids on metal oxides. The substances used are copper oxide and. there are three main steps for writing the net ionic equation for cuo + hcl = cucl2 + h2o (copper (ii) oxide +. It shows the reactants (substances that start a reaction) and products. this reaction is a type of. Copper 2 Oxide + Hydrochloric Acid.
From www.youtube.com
How to Write the Net Ionic Equation for CuO + HCl = CuCl2 + H2O YouTube Copper 2 Oxide + Hydrochloric Acid The substances used are copper oxide and. It shows the reactants (substances that start a reaction) and products. there are three main steps for writing the net ionic equation for cuo + hcl = cucl2 + h2o (copper (ii) oxide +. this video demonstrates the action of acids on metal oxides. a chemical equation represents a chemical. Copper 2 Oxide + Hydrochloric Acid.
From www.youtube.com
Copper & Hydrochloric Acid Ligand Substitution YouTube Copper 2 Oxide + Hydrochloric Acid a chemical equation represents a chemical reaction. activity 2.7 from chapter 2 of the ncert class 10 science textbook demonstrates the reaction between a. there are three main steps for writing the net ionic equation for cuo + hcl = cucl2 + h2o (copper (ii) oxide +. cuo + hcl = cucl2 + h2o is a. Copper 2 Oxide + Hydrochloric Acid.
From www.sciencephoto.com
Copper (II) oxide reacts with hydrochloric acid Stock Image C036 Copper 2 Oxide + Hydrochloric Acid The substances used are copper oxide and. this reaction is a type of double replacement reaction called a neutralization reaction, in which the solid. there are three main steps for writing the net ionic equation for cuo + hcl = cucl2 + h2o (copper (ii) oxide +. this video demonstrates the action of acids on metal oxides.. Copper 2 Oxide + Hydrochloric Acid.
From www.sciencephoto.com
Copper (II) oxide Stock Image C007/0865 Science Photo Library Copper 2 Oxide + Hydrochloric Acid this video demonstrates the action of acids on metal oxides. It shows the reactants (substances that start a reaction) and products. cuo + hcl = cucl2 + h2o is a double displacement (metathesis) reaction where one mole of copper(ii) oxide [cuo] and two. The substances used are copper oxide and. activity 2.7 from chapter 2 of the. Copper 2 Oxide + Hydrochloric Acid.
From www.sciencephoto.com
Two copper oxide reactions Stock Image A500/0465 Science Photo Library Copper 2 Oxide + Hydrochloric Acid It shows the reactants (substances that start a reaction) and products. The substances used are copper oxide and. activity 2.7 from chapter 2 of the ncert class 10 science textbook demonstrates the reaction between a. cuo + hcl = cucl2 + h2o is a double displacement (metathesis) reaction where one mole of copper(ii) oxide [cuo] and two. . Copper 2 Oxide + Hydrochloric Acid.
From www.youtube.com
Reaction of Metallic oxides YouTube Copper 2 Oxide + Hydrochloric Acid let us start with the reaction between copper (ii) oxide and hydrochloric acid. cuo + hcl = cucl2 + h2o is a double displacement (metathesis) reaction where one mole of copper(ii) oxide [cuo] and two. activity 2.7 from chapter 2 of the ncert class 10 science textbook demonstrates the reaction between a. The substances used are copper. Copper 2 Oxide + Hydrochloric Acid.
From www.youtube.com
Hydrochloric Acid Reaction HCL and mixed metal copper oxides YouTube Copper 2 Oxide + Hydrochloric Acid copper(ii) oxide or cupric oxide is an inorganic compound with the formula cuo. a chemical equation represents a chemical reaction. cuo + hcl = cucl2 + h2o is a double displacement (metathesis) reaction where one mole of copper(ii) oxide [cuo] and two. A black solid, it is one of the two stable. there are three main. Copper 2 Oxide + Hydrochloric Acid.
From exospybey.blob.core.windows.net
Copper Ii Oxide And Carbon Dioxide at Carmen Gadson blog Copper 2 Oxide + Hydrochloric Acid let us start with the reaction between copper (ii) oxide and hydrochloric acid. this video demonstrates the action of acids on metal oxides. cuo + hcl = cucl2 + h2o is a double displacement (metathesis) reaction where one mole of copper(ii) oxide [cuo] and two. copper(ii) oxide or cupric oxide is an inorganic compound with the. Copper 2 Oxide + Hydrochloric Acid.
From www.slideserve.com
PPT Laboratory 02 The Discovery of Chemical Change Through the Copper 2 Oxide + Hydrochloric Acid let us start with the reaction between copper (ii) oxide and hydrochloric acid. this video demonstrates the action of acids on metal oxides. The substances used are copper oxide and. a chemical equation represents a chemical reaction. cuo + hcl = cucl2 + h2o is a double displacement (metathesis) reaction where one mole of copper(ii) oxide. Copper 2 Oxide + Hydrochloric Acid.
From www.gkseries.com
When copper oxide and dilute hydrochloric acid react, colour changes to Copper 2 Oxide + Hydrochloric Acid a chemical equation represents a chemical reaction. cuo + hcl = cucl2 + h2o is a double displacement (metathesis) reaction where one mole of copper(ii) oxide [cuo] and two. this video demonstrates the action of acids on metal oxides. this reaction is a type of double replacement reaction called a neutralization reaction, in which the solid.. Copper 2 Oxide + Hydrochloric Acid.
From www.youtube.com
COPPER(II) CARBONATE & HYDROCHLORIC ACID DEMONSTRATION YouTube Copper 2 Oxide + Hydrochloric Acid activity 2.7 from chapter 2 of the ncert class 10 science textbook demonstrates the reaction between a. copper(ii) oxide or cupric oxide is an inorganic compound with the formula cuo. A black solid, it is one of the two stable. there are three main steps for writing the net ionic equation for cuo + hcl = cucl2. Copper 2 Oxide + Hydrochloric Acid.
From www.youtube.com
Reaction of Copper Oxide With Hydrochloric Acid YouTube Copper 2 Oxide + Hydrochloric Acid this video demonstrates the action of acids on metal oxides. cuo + hcl = cucl2 + h2o is a double displacement (metathesis) reaction where one mole of copper(ii) oxide [cuo] and two. The substances used are copper oxide and. In order for the products to form, the cation cu2+ from copper two oxide and the. activity 2.7. Copper 2 Oxide + Hydrochloric Acid.
From www.youtube.com
How to Balance CuO + HCl = CuCl2 + H2O YouTube Copper 2 Oxide + Hydrochloric Acid there are three main steps for writing the net ionic equation for cuo + hcl = cucl2 + h2o (copper (ii) oxide +. It shows the reactants (substances that start a reaction) and products. copper(ii) oxide or cupric oxide is an inorganic compound with the formula cuo. activity 2.7 from chapter 2 of the ncert class 10. Copper 2 Oxide + Hydrochloric Acid.
From www.sciencephoto.com
Copper in hydrochloric acid Stock Image A500/0755 Science Photo Copper 2 Oxide + Hydrochloric Acid copper(ii) oxide or cupric oxide is an inorganic compound with the formula cuo. there are three main steps for writing the net ionic equation for cuo + hcl = cucl2 + h2o (copper (ii) oxide +. A black solid, it is one of the two stable. this reaction is a type of double replacement reaction called a. Copper 2 Oxide + Hydrochloric Acid.
From www.numerade.com
SOLVED 6. Copper(II) oxide reacts with hydrochloric acid to produce Copper 2 Oxide + Hydrochloric Acid a chemical equation represents a chemical reaction. this video demonstrates the action of acids on metal oxides. A black solid, it is one of the two stable. copper(ii) oxide or cupric oxide is an inorganic compound with the formula cuo. The substances used are copper oxide and. In order for the products to form, the cation cu2+. Copper 2 Oxide + Hydrochloric Acid.
From www.youtube.com
How to write the equation for CuO + H2O Copper (II) oxide + Water Copper 2 Oxide + Hydrochloric Acid The substances used are copper oxide and. this reaction is a type of double replacement reaction called a neutralization reaction, in which the solid. this video demonstrates the action of acids on metal oxides. a chemical equation represents a chemical reaction. let us start with the reaction between copper (ii) oxide and hydrochloric acid. cuo. Copper 2 Oxide + Hydrochloric Acid.
From www.nagwa.com
Question Video Identifying the Products When a Metal Oxide Reacts with Copper 2 Oxide + Hydrochloric Acid It shows the reactants (substances that start a reaction) and products. In order for the products to form, the cation cu2+ from copper two oxide and the. this video demonstrates the action of acids on metal oxides. there are three main steps for writing the net ionic equation for cuo + hcl = cucl2 + h2o (copper (ii). Copper 2 Oxide + Hydrochloric Acid.
From www.sciencephoto.com
Copper in hydrochloric acid Stock Image A500/0621 Science Photo Copper 2 Oxide + Hydrochloric Acid there are three main steps for writing the net ionic equation for cuo + hcl = cucl2 + h2o (copper (ii) oxide +. copper(ii) oxide or cupric oxide is an inorganic compound with the formula cuo. cuo + hcl = cucl2 + h2o is a double displacement (metathesis) reaction where one mole of copper(ii) oxide [cuo] and. Copper 2 Oxide + Hydrochloric Acid.
From www.diomedia.com
STOCK IMAGE, , JF0694, 01B473E7 , Science Source Search Stock Photos Copper 2 Oxide + Hydrochloric Acid this reaction is a type of double replacement reaction called a neutralization reaction, in which the solid. The substances used are copper oxide and. In order for the products to form, the cation cu2+ from copper two oxide and the. this video demonstrates the action of acids on metal oxides. copper(ii) oxide or cupric oxide is an. Copper 2 Oxide + Hydrochloric Acid.
From www.youtube.com
Chemical Properties of Hydrogen Reaction of hydrogen with copper II Copper 2 Oxide + Hydrochloric Acid It shows the reactants (substances that start a reaction) and products. In order for the products to form, the cation cu2+ from copper two oxide and the. this video demonstrates the action of acids on metal oxides. this reaction is a type of double replacement reaction called a neutralization reaction, in which the solid. The substances used are. Copper 2 Oxide + Hydrochloric Acid.
From www.youtube.com
CuO+HCl=CuCl2+H2O Balanced EquationCopper oxide+Hydrochloric acid Copper 2 Oxide + Hydrochloric Acid In order for the products to form, the cation cu2+ from copper two oxide and the. a chemical equation represents a chemical reaction. this video demonstrates the action of acids on metal oxides. It shows the reactants (substances that start a reaction) and products. there are three main steps for writing the net ionic equation for cuo. Copper 2 Oxide + Hydrochloric Acid.