Bromide Bromate Activation Energy . Determine the activation energy for the reaction between bromide ions and bromate (v) ions. To use the arrhenius equation to determine the. Describe the steps to determining activation energy between bromide and bromate ions. Pipette 10 cm3 of phenol solution and 10 cm3. Effect of concentration on reaction rates rates of reaction are affected by. In this practical the activation energy for the reaction between bromide ions and bromate (v) ions will be determined. This laboratory experiment placed an emphasis on the determination of the order of reaction and ultimately the rate constant and activation energy. The reaction between bromide and bromate ions in acid solution is a slow chemical reaction at room temperature. In this practical the activation energy for the reaction between bromide ions and bromate (v) ions will be determined.
from us.metoree.com
Describe the steps to determining activation energy between bromide and bromate ions. Pipette 10 cm3 of phenol solution and 10 cm3. Determine the activation energy for the reaction between bromide ions and bromate (v) ions. In this practical the activation energy for the reaction between bromide ions and bromate (v) ions will be determined. This laboratory experiment placed an emphasis on the determination of the order of reaction and ultimately the rate constant and activation energy. Effect of concentration on reaction rates rates of reaction are affected by. In this practical the activation energy for the reaction between bromide ions and bromate (v) ions will be determined. The reaction between bromide and bromate ions in acid solution is a slow chemical reaction at room temperature. To use the arrhenius equation to determine the.
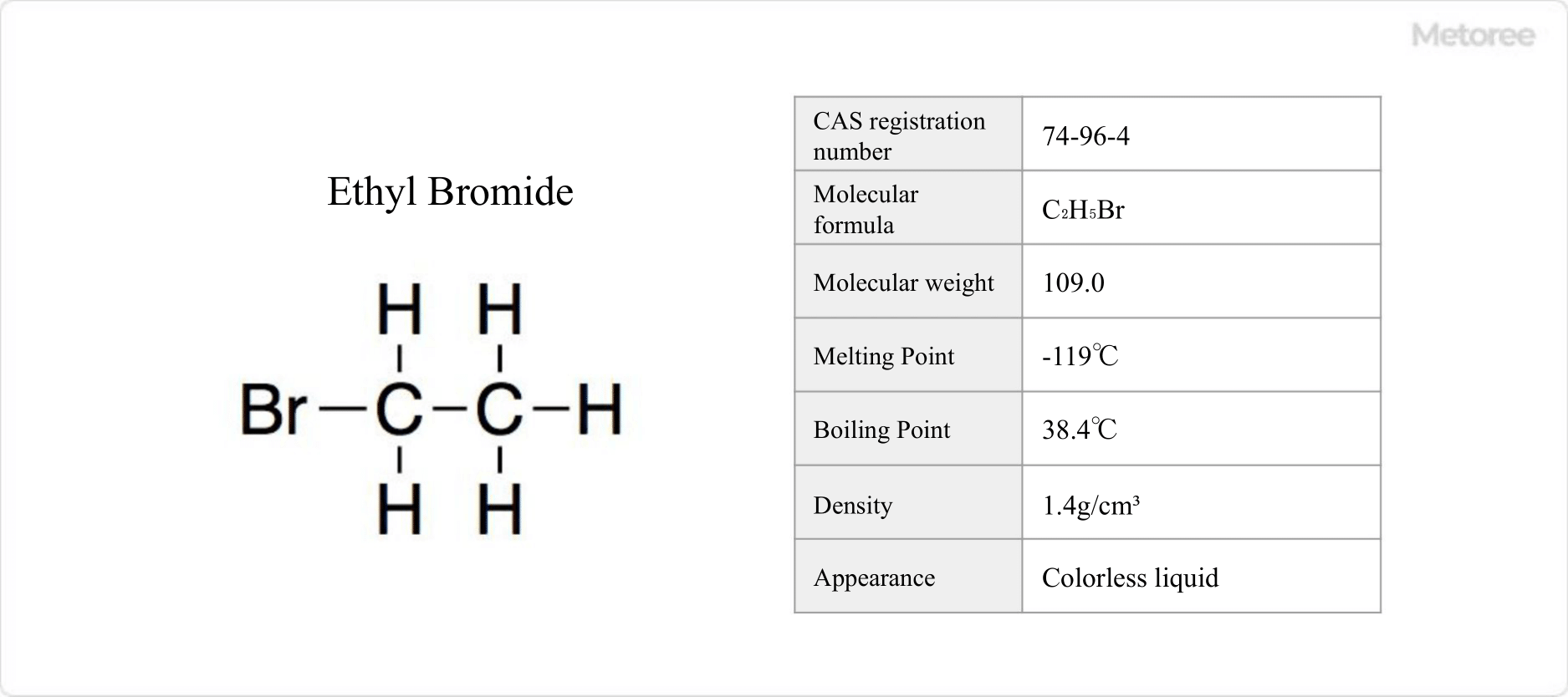
41 Ethyl Bromide Manufacturers in 2024 Metoree
Bromide Bromate Activation Energy To use the arrhenius equation to determine the. In this practical the activation energy for the reaction between bromide ions and bromate (v) ions will be determined. Pipette 10 cm3 of phenol solution and 10 cm3. In this practical the activation energy for the reaction between bromide ions and bromate (v) ions will be determined. Effect of concentration on reaction rates rates of reaction are affected by. This laboratory experiment placed an emphasis on the determination of the order of reaction and ultimately the rate constant and activation energy. The reaction between bromide and bromate ions in acid solution is a slow chemical reaction at room temperature. To use the arrhenius equation to determine the. Describe the steps to determining activation energy between bromide and bromate ions. Determine the activation energy for the reaction between bromide ions and bromate (v) ions.
From www.chegg.com
Solved The reaction between bromate and bromide in an acid Bromide Bromate Activation Energy In this practical the activation energy for the reaction between bromide ions and bromate (v) ions will be determined. Determine the activation energy for the reaction between bromide ions and bromate (v) ions. Describe the steps to determining activation energy between bromide and bromate ions. In this practical the activation energy for the reaction between bromide ions and bromate (v). Bromide Bromate Activation Energy.
From www.studypool.com
SOLUTION To determine the activation energy of the reaction between Bromide Bromate Activation Energy Effect of concentration on reaction rates rates of reaction are affected by. Describe the steps to determining activation energy between bromide and bromate ions. This laboratory experiment placed an emphasis on the determination of the order of reaction and ultimately the rate constant and activation energy. Pipette 10 cm3 of phenol solution and 10 cm3. In this practical the activation. Bromide Bromate Activation Energy.
From www.chegg.com
Solved The reaction between bromate and bromide in an acid Bromide Bromate Activation Energy Effect of concentration on reaction rates rates of reaction are affected by. Pipette 10 cm3 of phenol solution and 10 cm3. This laboratory experiment placed an emphasis on the determination of the order of reaction and ultimately the rate constant and activation energy. In this practical the activation energy for the reaction between bromide ions and bromate (v) ions will. Bromide Bromate Activation Energy.
From www.bhphotovideo.com
Photographers' Formulary Potassium Bromide (100g) 100930 100G Bromide Bromate Activation Energy Pipette 10 cm3 of phenol solution and 10 cm3. Determine the activation energy for the reaction between bromide ions and bromate (v) ions. In this practical the activation energy for the reaction between bromide ions and bromate (v) ions will be determined. In this practical the activation energy for the reaction between bromide ions and bromate (v) ions will be. Bromide Bromate Activation Energy.
From www.markedbyteachers.com
To Determine the Activation Energy of the Reaction between Bromide ion Bromide Bromate Activation Energy To use the arrhenius equation to determine the. This laboratory experiment placed an emphasis on the determination of the order of reaction and ultimately the rate constant and activation energy. Determine the activation energy for the reaction between bromide ions and bromate (v) ions. Pipette 10 cm3 of phenol solution and 10 cm3. Effect of concentration on reaction rates rates. Bromide Bromate Activation Energy.
From www.chemistryworld.com
Potassium bromide Podcast Chemistry World Bromide Bromate Activation Energy Pipette 10 cm3 of phenol solution and 10 cm3. Describe the steps to determining activation energy between bromide and bromate ions. In this practical the activation energy for the reaction between bromide ions and bromate (v) ions will be determined. Effect of concentration on reaction rates rates of reaction are affected by. To use the arrhenius equation to determine the.. Bromide Bromate Activation Energy.
From www.numerade.com
What is the difference between (a) a bromine atom, (b) a bromine Bromide Bromate Activation Energy The reaction between bromide and bromate ions in acid solution is a slow chemical reaction at room temperature. This laboratory experiment placed an emphasis on the determination of the order of reaction and ultimately the rate constant and activation energy. In this practical the activation energy for the reaction between bromide ions and bromate (v) ions will be determined. Describe. Bromide Bromate Activation Energy.
From www.fishersci.com
Bromoacetyl bromide, 98, Thermo Scientific Chemicals, Quantity 25 g Bromide Bromate Activation Energy Describe the steps to determining activation energy between bromide and bromate ions. Effect of concentration on reaction rates rates of reaction are affected by. In this practical the activation energy for the reaction between bromide ions and bromate (v) ions will be determined. Determine the activation energy for the reaction between bromide ions and bromate (v) ions. Pipette 10 cm3. Bromide Bromate Activation Energy.
From www.chegg.com
Solved The reaction between bromate and bromide in an acid Bromide Bromate Activation Energy In this practical the activation energy for the reaction between bromide ions and bromate (v) ions will be determined. This laboratory experiment placed an emphasis on the determination of the order of reaction and ultimately the rate constant and activation energy. In this practical the activation energy for the reaction between bromide ions and bromate (v) ions will be determined.. Bromide Bromate Activation Energy.
From www.studypool.com
SOLUTION To determine the activation energy of the reaction between Bromide Bromate Activation Energy This laboratory experiment placed an emphasis on the determination of the order of reaction and ultimately the rate constant and activation energy. Determine the activation energy for the reaction between bromide ions and bromate (v) ions. Effect of concentration on reaction rates rates of reaction are affected by. The reaction between bromide and bromate ions in acid solution is a. Bromide Bromate Activation Energy.
From www.studypool.com
SOLUTION Aim to determine the activation energy of the reaction Bromide Bromate Activation Energy The reaction between bromide and bromate ions in acid solution is a slow chemical reaction at room temperature. Pipette 10 cm3 of phenol solution and 10 cm3. To use the arrhenius equation to determine the. In this practical the activation energy for the reaction between bromide ions and bromate (v) ions will be determined. Effect of concentration on reaction rates. Bromide Bromate Activation Energy.
From www.differencebetween.com
Difference Between Bromine and Bromide Compare the Difference Between Bromide Bromate Activation Energy This laboratory experiment placed an emphasis on the determination of the order of reaction and ultimately the rate constant and activation energy. Describe the steps to determining activation energy between bromide and bromate ions. In this practical the activation energy for the reaction between bromide ions and bromate (v) ions will be determined. In this practical the activation energy for. Bromide Bromate Activation Energy.
From us.metoree.com
41 Ethyl Bromide Manufacturers in 2024 Metoree Bromide Bromate Activation Energy Pipette 10 cm3 of phenol solution and 10 cm3. Effect of concentration on reaction rates rates of reaction are affected by. Determine the activation energy for the reaction between bromide ions and bromate (v) ions. In this practical the activation energy for the reaction between bromide ions and bromate (v) ions will be determined. Describe the steps to determining activation. Bromide Bromate Activation Energy.
From www.rpicorp.com
E718001.0 Ethidium Bromide, Powder, 1 Gram Bromide Bromate Activation Energy To use the arrhenius equation to determine the. Effect of concentration on reaction rates rates of reaction are affected by. The reaction between bromide and bromate ions in acid solution is a slow chemical reaction at room temperature. In this practical the activation energy for the reaction between bromide ions and bromate (v) ions will be determined. This laboratory experiment. Bromide Bromate Activation Energy.
From www.drsebiscellfood.com
Bromide Plus Powder Dr. Sebi's Cell Food Bromide Bromate Activation Energy Determine the activation energy for the reaction between bromide ions and bromate (v) ions. In this practical the activation energy for the reaction between bromide ions and bromate (v) ions will be determined. Pipette 10 cm3 of phenol solution and 10 cm3. This laboratory experiment placed an emphasis on the determination of the order of reaction and ultimately the rate. Bromide Bromate Activation Energy.
From www.youtube.com
F5147 E43 06 Determining the activation energy of the reaction between Bromide Bromate Activation Energy The reaction between bromide and bromate ions in acid solution is a slow chemical reaction at room temperature. In this practical the activation energy for the reaction between bromide ions and bromate (v) ions will be determined. This laboratory experiment placed an emphasis on the determination of the order of reaction and ultimately the rate constant and activation energy. Effect. Bromide Bromate Activation Energy.
From www.studypool.com
SOLUTION To determine the activation energy of the reaction between Bromide Bromate Activation Energy In this practical the activation energy for the reaction between bromide ions and bromate (v) ions will be determined. The reaction between bromide and bromate ions in acid solution is a slow chemical reaction at room temperature. Pipette 10 cm3 of phenol solution and 10 cm3. Effect of concentration on reaction rates rates of reaction are affected by. Describe the. Bromide Bromate Activation Energy.
From www.nanochemazone.com
1Dodecyl3methylimidazolium Bromide Low Price 45 High Purity Bromide Bromate Activation Energy The reaction between bromide and bromate ions in acid solution is a slow chemical reaction at room temperature. In this practical the activation energy for the reaction between bromide ions and bromate (v) ions will be determined. Determine the activation energy for the reaction between bromide ions and bromate (v) ions. To use the arrhenius equation to determine the. This. Bromide Bromate Activation Energy.
From byjus.com
Write the molecular formulae for the compound Copper II Bromide Bromide Bromate Activation Energy Describe the steps to determining activation energy between bromide and bromate ions. To use the arrhenius equation to determine the. Determine the activation energy for the reaction between bromide ions and bromate (v) ions. This laboratory experiment placed an emphasis on the determination of the order of reaction and ultimately the rate constant and activation energy. In this practical the. Bromide Bromate Activation Energy.
From www.studypool.com
SOLUTION Experiment 10f chemistry tas lab report to determine the Bromide Bromate Activation Energy To use the arrhenius equation to determine the. Determine the activation energy for the reaction between bromide ions and bromate (v) ions. In this practical the activation energy for the reaction between bromide ions and bromate (v) ions will be determined. The reaction between bromide and bromate ions in acid solution is a slow chemical reaction at room temperature. In. Bromide Bromate Activation Energy.
From www.studypool.com
SOLUTION Aim to determine the activation energy of the reaction Bromide Bromate Activation Energy In this practical the activation energy for the reaction between bromide ions and bromate (v) ions will be determined. To use the arrhenius equation to determine the. The reaction between bromide and bromate ions in acid solution is a slow chemical reaction at room temperature. Describe the steps to determining activation energy between bromide and bromate ions. In this practical. Bromide Bromate Activation Energy.
From www.youtube.com
Sodium bromide YouTube Bromide Bromate Activation Energy Determine the activation energy for the reaction between bromide ions and bromate (v) ions. In this practical the activation energy for the reaction between bromide ions and bromate (v) ions will be determined. In this practical the activation energy for the reaction between bromide ions and bromate (v) ions will be determined. The reaction between bromide and bromate ions in. Bromide Bromate Activation Energy.
From www.chemkits.eu
Sodium bromide, 99.8+, 7647156 Bromide Bromate Activation Energy To use the arrhenius equation to determine the. In this practical the activation energy for the reaction between bromide ions and bromate (v) ions will be determined. Describe the steps to determining activation energy between bromide and bromate ions. Pipette 10 cm3 of phenol solution and 10 cm3. The reaction between bromide and bromate ions in acid solution is a. Bromide Bromate Activation Energy.
From www.scribd.com
Experiment 1_bromate bromide reaction PDF Bromide Bromate Activation Energy Pipette 10 cm3 of phenol solution and 10 cm3. The reaction between bromide and bromate ions in acid solution is a slow chemical reaction at room temperature. To use the arrhenius equation to determine the. In this practical the activation energy for the reaction between bromide ions and bromate (v) ions will be determined. Determine the activation energy for the. Bromide Bromate Activation Energy.
From www.studypool.com
SOLUTION To determine the activation energy of the reaction between Bromide Bromate Activation Energy In this practical the activation energy for the reaction between bromide ions and bromate (v) ions will be determined. To use the arrhenius equation to determine the. The reaction between bromide and bromate ions in acid solution is a slow chemical reaction at room temperature. This laboratory experiment placed an emphasis on the determination of the order of reaction and. Bromide Bromate Activation Energy.
From www.numerade.com
SOLVED 'QUESTION 8 Aluminum will react with bromine to form aluminum Bromide Bromate Activation Energy Effect of concentration on reaction rates rates of reaction are affected by. Describe the steps to determining activation energy between bromide and bromate ions. To use the arrhenius equation to determine the. In this practical the activation energy for the reaction between bromide ions and bromate (v) ions will be determined. Determine the activation energy for the reaction between bromide. Bromide Bromate Activation Energy.
From www.studypool.com
SOLUTION Experiment 10f chemistry tas lab report to determine the Bromide Bromate Activation Energy Pipette 10 cm3 of phenol solution and 10 cm3. Effect of concentration on reaction rates rates of reaction are affected by. In this practical the activation energy for the reaction between bromide ions and bromate (v) ions will be determined. In this practical the activation energy for the reaction between bromide ions and bromate (v) ions will be determined. To. Bromide Bromate Activation Energy.
From www.youtube.com
Ethene + Hydrogen Bromide (Reaction and Mechanism) YouTube Bromide Bromate Activation Energy This laboratory experiment placed an emphasis on the determination of the order of reaction and ultimately the rate constant and activation energy. In this practical the activation energy for the reaction between bromide ions and bromate (v) ions will be determined. Describe the steps to determining activation energy between bromide and bromate ions. Pipette 10 cm3 of phenol solution and. Bromide Bromate Activation Energy.
From www.studypool.com
SOLUTION Determining the activation energy for the reaction between Bromide Bromate Activation Energy Describe the steps to determining activation energy between bromide and bromate ions. Effect of concentration on reaction rates rates of reaction are affected by. To use the arrhenius equation to determine the. Determine the activation energy for the reaction between bromide ions and bromate (v) ions. In this practical the activation energy for the reaction between bromide ions and bromate. Bromide Bromate Activation Energy.
From www.studypool.com
SOLUTION Determining the activation energy for the reaction between Bromide Bromate Activation Energy Effect of concentration on reaction rates rates of reaction are affected by. Describe the steps to determining activation energy between bromide and bromate ions. To use the arrhenius equation to determine the. Pipette 10 cm3 of phenol solution and 10 cm3. In this practical the activation energy for the reaction between bromide ions and bromate (v) ions will be determined.. Bromide Bromate Activation Energy.
From pubs.acs.org
A New Reaction Pathway for Bromite to Bromate in the Ozonation of Bromide Bromate Activation Energy Determine the activation energy for the reaction between bromide ions and bromate (v) ions. Effect of concentration on reaction rates rates of reaction are affected by. The reaction between bromide and bromate ions in acid solution is a slow chemical reaction at room temperature. To use the arrhenius equation to determine the. This laboratory experiment placed an emphasis on the. Bromide Bromate Activation Energy.
From sielc.com
Vinyl bromide SIELC Technologies Bromide Bromate Activation Energy Describe the steps to determining activation energy between bromide and bromate ions. In this practical the activation energy for the reaction between bromide ions and bromate (v) ions will be determined. In this practical the activation energy for the reaction between bromide ions and bromate (v) ions will be determined. Determine the activation energy for the reaction between bromide ions. Bromide Bromate Activation Energy.
From www.studypool.com
SOLUTION Determining the activation energy for the reaction between Bromide Bromate Activation Energy In this practical the activation energy for the reaction between bromide ions and bromate (v) ions will be determined. Pipette 10 cm3 of phenol solution and 10 cm3. To use the arrhenius equation to determine the. Effect of concentration on reaction rates rates of reaction are affected by. Describe the steps to determining activation energy between bromide and bromate ions.. Bromide Bromate Activation Energy.
From www.studypool.com
SOLUTION To determine the activation energy of the reaction between Bromide Bromate Activation Energy Describe the steps to determining activation energy between bromide and bromate ions. In this practical the activation energy for the reaction between bromide ions and bromate (v) ions will be determined. This laboratory experiment placed an emphasis on the determination of the order of reaction and ultimately the rate constant and activation energy. Determine the activation energy for the reaction. Bromide Bromate Activation Energy.
From www.dreamstime.com
3D Image of Vinyl Bromide Skeletal Formula Stock Illustration Bromide Bromate Activation Energy Determine the activation energy for the reaction between bromide ions and bromate (v) ions. This laboratory experiment placed an emphasis on the determination of the order of reaction and ultimately the rate constant and activation energy. In this practical the activation energy for the reaction between bromide ions and bromate (v) ions will be determined. In this practical the activation. Bromide Bromate Activation Energy.