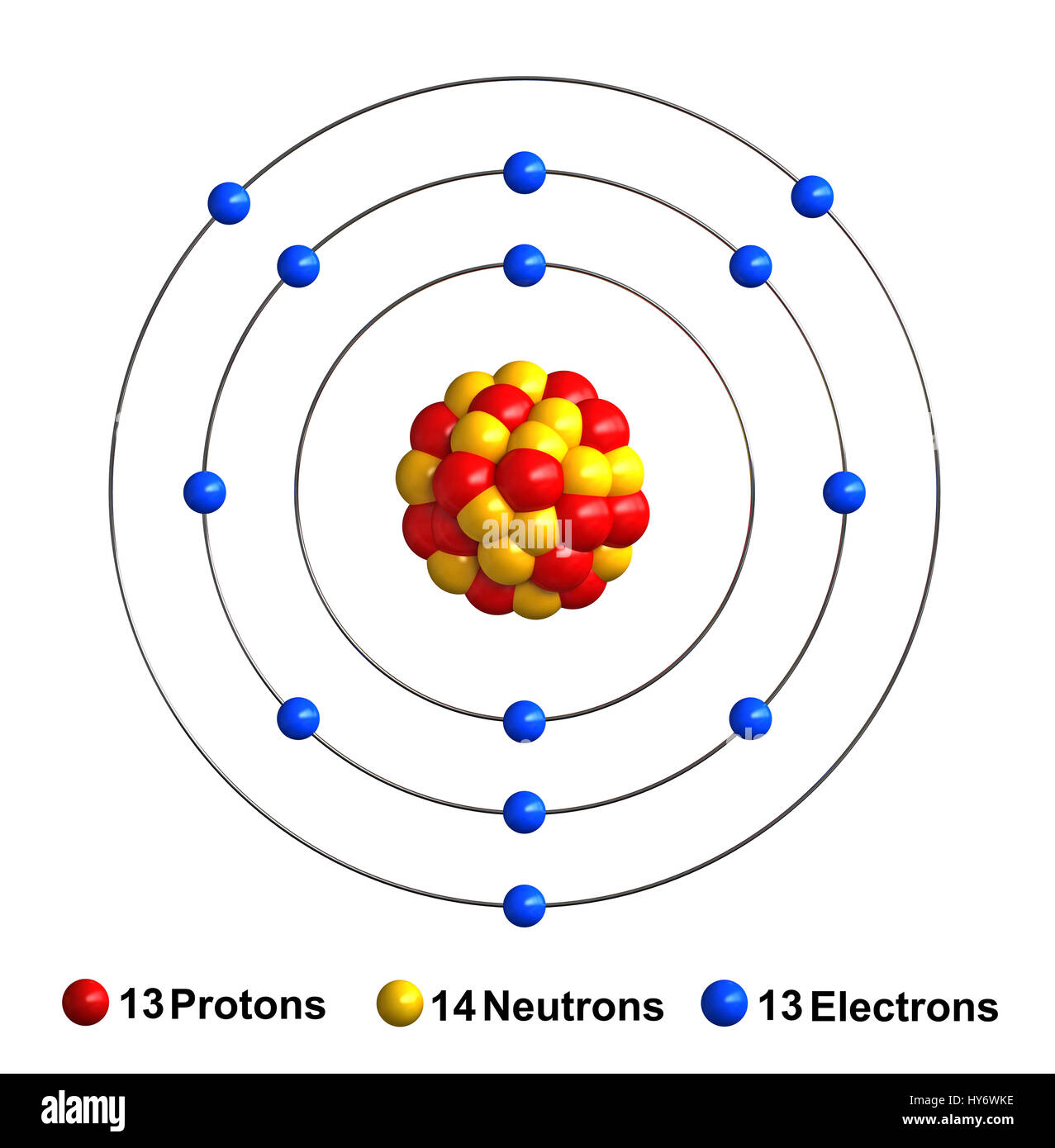
Aluminum Atom Have Valence Electrons . However, one atom only has two electrons, so it could never have more than 2 valence electrons. But for most of the transition. An aluminum atom has three valence electrons. How many valence electrons are in one atom of each element? Aluminum is the third element in the third row of the periodic table. What happens to aluminum when it reacts with. Write the configuration of aluminum, assuming that it has lost its valence electrons. Sulfur (s) is located in group via (group 16), so it has 6 valence electrons. The outermost orbitals to be filled are 3s and 3p. Electron configuration of aluminium is [ne] 3s2 3p1. Helium (he) is located in group viiia (group 18). The number of electrons in each element’s electron shells, particularly the outermost valence shell, is the primary factor in determining its chemical bonding behavior. In the periodic table, the elements are listed in order of increasing atomic number z. 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or form—are those that. Do you think it will lose three electrons or gain five electrons to obtain an octet in its.
from www.alamy.com
Write the configuration of aluminum, assuming that it has lost its valence electrons. However, one atom only has two electrons, so it could never have more than 2 valence electrons. How many valence electrons are in one atom of each element? 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or form—are those that. The outermost orbitals to be filled are 3s and 3p. Helium (he) is located in group viiia (group 18). The number of electrons in each element’s electron shells, particularly the outermost valence shell, is the primary factor in determining its chemical bonding behavior. But for most of the transition. Do you think it will lose three electrons or gain five electrons to obtain an octet in its. Sulfur (s) is located in group via (group 16), so it has 6 valence electrons.
3d render of atom structure of aluminum isolated over white background
Aluminum Atom Have Valence Electrons Aluminum is the third element in the third row of the periodic table. How many valence electrons are in one atom of each element? What happens to aluminum when it reacts with. Sulfur (s) is located in group via (group 16), so it has 6 valence electrons. Do you think it will lose three electrons or gain five electrons to obtain an octet in its. The outermost orbitals to be filled are 3s and 3p. Aluminum is the third element in the third row of the periodic table. However, one atom only has two electrons, so it could never have more than 2 valence electrons. Electron configuration of aluminium is [ne] 3s2 3p1. But for most of the transition. An aluminum atom has three valence electrons. Write the configuration of aluminum, assuming that it has lost its valence electrons. In the periodic table, the elements are listed in order of increasing atomic number z. The number of electrons in each element’s electron shells, particularly the outermost valence shell, is the primary factor in determining its chemical bonding behavior. 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or form—are those that. Helium (he) is located in group viiia (group 18).
From www.alamy.com
3d render of atom structure of aluminum isolated over white background Aluminum Atom Have Valence Electrons Sulfur (s) is located in group via (group 16), so it has 6 valence electrons. Do you think it will lose three electrons or gain five electrons to obtain an octet in its. But for most of the transition. How many valence electrons are in one atom of each element? However, one atom only has two electrons, so it could. Aluminum Atom Have Valence Electrons.
From www.alamy.com
Aluminium (Al). Diagram of the nuclear composition and electron Aluminum Atom Have Valence Electrons An aluminum atom has three valence electrons. The outermost orbitals to be filled are 3s and 3p. 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or form—are those that. In the periodic table, the elements are listed in order of increasing atomic number z. What happens to aluminum. Aluminum Atom Have Valence Electrons.
From sciencenotes.org
What Are Valence Electrons? Definition and Periodic Table Aluminum Atom Have Valence Electrons Write the configuration of aluminum, assuming that it has lost its valence electrons. Sulfur (s) is located in group via (group 16), so it has 6 valence electrons. The outermost orbitals to be filled are 3s and 3p. What happens to aluminum when it reacts with. In the periodic table, the elements are listed in order of increasing atomic number. Aluminum Atom Have Valence Electrons.
From valenceelectrons.com
How many valence electrons does aluminum(Al) have? Aluminum Atom Have Valence Electrons The number of electrons in each element’s electron shells, particularly the outermost valence shell, is the primary factor in determining its chemical bonding behavior. Do you think it will lose three electrons or gain five electrons to obtain an octet in its. The outermost orbitals to be filled are 3s and 3p. Aluminum is the third element in the third. Aluminum Atom Have Valence Electrons.
From ar.inspiredpencil.com
Aluminium Atomic Structure Aluminum Atom Have Valence Electrons How many valence electrons are in one atom of each element? An aluminum atom has three valence electrons. The outermost orbitals to be filled are 3s and 3p. Electron configuration of aluminium is [ne] 3s2 3p1. 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or form—are those that.. Aluminum Atom Have Valence Electrons.
From material-properties.org
Aluminium Protons Neutrons Electrons Electron Configuration Aluminum Atom Have Valence Electrons Helium (he) is located in group viiia (group 18). Electron configuration of aluminium is [ne] 3s2 3p1. An aluminum atom has three valence electrons. 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or form—are those that. But for most of the transition. In the periodic table, the elements. Aluminum Atom Have Valence Electrons.
From www.nagwa.com
Question Video Determining the Number of Electrons in the Valence Aluminum Atom Have Valence Electrons Electron configuration of aluminium is [ne] 3s2 3p1. An aluminum atom has three valence electrons. Write the configuration of aluminum, assuming that it has lost its valence electrons. The outermost orbitals to be filled are 3s and 3p. Aluminum is the third element in the third row of the periodic table. 93 rows you may assume the valences of the. Aluminum Atom Have Valence Electrons.
From periodictable.me
How Can We Find Electron Configuration For Aluminium (Al) Aluminum Atom Have Valence Electrons Do you think it will lose three electrons or gain five electrons to obtain an octet in its. In the periodic table, the elements are listed in order of increasing atomic number z. Write the configuration of aluminum, assuming that it has lost its valence electrons. Sulfur (s) is located in group via (group 16), so it has 6 valence. Aluminum Atom Have Valence Electrons.
From www.alamy.com
Aluminium (Al). Diagram of the nuclear composition, electron Aluminum Atom Have Valence Electrons How many valence electrons are in one atom of each element? The number of electrons in each element’s electron shells, particularly the outermost valence shell, is the primary factor in determining its chemical bonding behavior. Sulfur (s) is located in group via (group 16), so it has 6 valence electrons. What happens to aluminum when it reacts with. Write the. Aluminum Atom Have Valence Electrons.
From valenceelectrons.com
Protons, Neutrons, Electrons for Aluminum (Al, Al3+) Aluminum Atom Have Valence Electrons What happens to aluminum when it reacts with. However, one atom only has two electrons, so it could never have more than 2 valence electrons. Sulfur (s) is located in group via (group 16), so it has 6 valence electrons. An aluminum atom has three valence electrons. How many valence electrons are in one atom of each element? In the. Aluminum Atom Have Valence Electrons.
From www.museoinclusivo.com
Exploring Aluminum A Comprehensive Guide to Its Valence Electrons Aluminum Atom Have Valence Electrons Helium (he) is located in group viiia (group 18). How many valence electrons are in one atom of each element? Electron configuration of aluminium is [ne] 3s2 3p1. However, one atom only has two electrons, so it could never have more than 2 valence electrons. The number of electrons in each element’s electron shells, particularly the outermost valence shell, is. Aluminum Atom Have Valence Electrons.
From valenceelectrons.com
How Many Valence Electrons Does Aluminum (Al) Have? Aluminum Atom Have Valence Electrons An aluminum atom has three valence electrons. In the periodic table, the elements are listed in order of increasing atomic number z. Sulfur (s) is located in group via (group 16), so it has 6 valence electrons. The number of electrons in each element’s electron shells, particularly the outermost valence shell, is the primary factor in determining its chemical bonding. Aluminum Atom Have Valence Electrons.
From slideplayer.com
Bohr Diagrams Find your element on the periodic table. ppt download Aluminum Atom Have Valence Electrons In the periodic table, the elements are listed in order of increasing atomic number z. The outermost orbitals to be filled are 3s and 3p. But for most of the transition. Electron configuration of aluminium is [ne] 3s2 3p1. What happens to aluminum when it reacts with. Sulfur (s) is located in group via (group 16), so it has 6. Aluminum Atom Have Valence Electrons.
From www.vectorstock.com
Diagram representation of the element aluminium Vector Image Aluminum Atom Have Valence Electrons But for most of the transition. 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or form—are those that. Electron configuration of aluminium is [ne] 3s2 3p1. An aluminum atom has three valence electrons. The number of electrons in each element’s electron shells, particularly the outermost valence shell, is. Aluminum Atom Have Valence Electrons.
From www.newtondesk.com
Aluminium Al (Element 13) of Periodic Table Elements FlashCards Aluminum Atom Have Valence Electrons The outermost orbitals to be filled are 3s and 3p. What happens to aluminum when it reacts with. The number of electrons in each element’s electron shells, particularly the outermost valence shell, is the primary factor in determining its chemical bonding behavior. Write the configuration of aluminum, assuming that it has lost its valence electrons. An aluminum atom has three. Aluminum Atom Have Valence Electrons.
From www.museoinclusivo.com
Exploring Aluminum A Comprehensive Guide to Its Valence Electrons Aluminum Atom Have Valence Electrons However, one atom only has two electrons, so it could never have more than 2 valence electrons. The outermost orbitals to be filled are 3s and 3p. An aluminum atom has three valence electrons. 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or form—are those that. Sulfur (s). Aluminum Atom Have Valence Electrons.
From www.youtube.com
How many valence electrons does aluminum have?How to find the valence Aluminum Atom Have Valence Electrons 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or form—are those that. How many valence electrons are in one atom of each element? The number of electrons in each element’s electron shells, particularly the outermost valence shell, is the primary factor in determining its chemical bonding behavior. But. Aluminum Atom Have Valence Electrons.
From www.youtube.com
How to Find Valence Electrons for Aluminum (Al) YouTube Aluminum Atom Have Valence Electrons Aluminum is the third element in the third row of the periodic table. How many valence electrons are in one atom of each element? Sulfur (s) is located in group via (group 16), so it has 6 valence electrons. Electron configuration of aluminium is [ne] 3s2 3p1. 93 rows you may assume the valences of the chemical elements—the number of. Aluminum Atom Have Valence Electrons.
From sciencenotes.org
Aluminum Atom Science Notes and Projects Aluminum Atom Have Valence Electrons 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or form—are those that. However, one atom only has two electrons, so it could never have more than 2 valence electrons. An aluminum atom has three valence electrons. Electron configuration of aluminium is [ne] 3s2 3p1. In the periodic table,. Aluminum Atom Have Valence Electrons.
From www.benjamin-mills.com
Electron arrangements Aluminum Atom Have Valence Electrons Do you think it will lose three electrons or gain five electrons to obtain an octet in its. Write the configuration of aluminum, assuming that it has lost its valence electrons. However, one atom only has two electrons, so it could never have more than 2 valence electrons. Aluminum is the third element in the third row of the periodic. Aluminum Atom Have Valence Electrons.
From valenceelectrons.com
How Many Valence Electrons Does Aluminum (Al) Have? Aluminum Atom Have Valence Electrons However, one atom only has two electrons, so it could never have more than 2 valence electrons. Aluminum is the third element in the third row of the periodic table. Electron configuration of aluminium is [ne] 3s2 3p1. But for most of the transition. What happens to aluminum when it reacts with. Helium (he) is located in group viiia (group. Aluminum Atom Have Valence Electrons.
From smk-tpz-web-api-1325663342.ap-south-1.elb.amazonaws.com
Al Aluminium Element Information Facts, Properties, Trends, Uses and Aluminum Atom Have Valence Electrons Do you think it will lose three electrons or gain five electrons to obtain an octet in its. Aluminum is the third element in the third row of the periodic table. Sulfur (s) is located in group via (group 16), so it has 6 valence electrons. But for most of the transition. An aluminum atom has three valence electrons. The. Aluminum Atom Have Valence Electrons.
From valenceelectrons.com
How to Find the Valence Electrons for Aluminum (Al)? Aluminum Atom Have Valence Electrons Aluminum is the third element in the third row of the periodic table. Write the configuration of aluminum, assuming that it has lost its valence electrons. But for most of the transition. How many valence electrons are in one atom of each element? Do you think it will lose three electrons or gain five electrons to obtain an octet in. Aluminum Atom Have Valence Electrons.
From cabinet.matttroy.net
Aluminum Periodic Table Protons Neutrons And Electrons Matttroy Aluminum Atom Have Valence Electrons An aluminum atom has three valence electrons. But for most of the transition. Helium (he) is located in group viiia (group 18). Aluminum is the third element in the third row of the periodic table. How many valence electrons are in one atom of each element? Write the configuration of aluminum, assuming that it has lost its valence electrons. Electron. Aluminum Atom Have Valence Electrons.
From valenceelectrons.com
How many valence electrons does aluminum(Al) have? Aluminum Atom Have Valence Electrons Aluminum is the third element in the third row of the periodic table. However, one atom only has two electrons, so it could never have more than 2 valence electrons. How many valence electrons are in one atom of each element? What happens to aluminum when it reacts with. The outermost orbitals to be filled are 3s and 3p. The. Aluminum Atom Have Valence Electrons.
From periodictable.me
Aluminum Valence Electrons Aluminum Valency (Al) with Dot Diagram Aluminum Atom Have Valence Electrons Write the configuration of aluminum, assuming that it has lost its valence electrons. Sulfur (s) is located in group via (group 16), so it has 6 valence electrons. Electron configuration of aluminium is [ne] 3s2 3p1. Helium (he) is located in group viiia (group 18). 93 rows you may assume the valences of the chemical elements—the number of electrons with. Aluminum Atom Have Valence Electrons.
From www.museoinclusivo.com
How Many Electrons Does an Aluminum Atom Have? Exploring the Atomic Aluminum Atom Have Valence Electrons An aluminum atom has three valence electrons. In the periodic table, the elements are listed in order of increasing atomic number z. Write the configuration of aluminum, assuming that it has lost its valence electrons. Helium (he) is located in group viiia (group 18). Electron configuration of aluminium is [ne] 3s2 3p1. Do you think it will lose three electrons. Aluminum Atom Have Valence Electrons.
From topblogtenz.com
Aluminum Orbital diagram, Electron configuration, and Valence electrons Aluminum Atom Have Valence Electrons Sulfur (s) is located in group via (group 16), so it has 6 valence electrons. Electron configuration of aluminium is [ne] 3s2 3p1. An aluminum atom has three valence electrons. How many valence electrons are in one atom of each element? In the periodic table, the elements are listed in order of increasing atomic number z. The number of electrons. Aluminum Atom Have Valence Electrons.
From mavink.com
Aluminum Shell Diagram Aluminum Atom Have Valence Electrons Helium (he) is located in group viiia (group 18). 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or form—are those that. The number of electrons in each element’s electron shells, particularly the outermost valence shell, is the primary factor in determining its chemical bonding behavior. An aluminum atom. Aluminum Atom Have Valence Electrons.
From www.museoinclusivo.com
How Many Valence Electrons Does Aluminum Have? Exploring the Atomic Aluminum Atom Have Valence Electrons The number of electrons in each element’s electron shells, particularly the outermost valence shell, is the primary factor in determining its chemical bonding behavior. Helium (he) is located in group viiia (group 18). Do you think it will lose three electrons or gain five electrons to obtain an octet in its. What happens to aluminum when it reacts with. How. Aluminum Atom Have Valence Electrons.
From www.clipartmax.com
Diagram, Atom, Iron, Atomic, Bohr, Nucleus Many Valence Electrons Aluminum Atom Have Valence Electrons An aluminum atom has three valence electrons. Helium (he) is located in group viiia (group 18). Electron configuration of aluminium is [ne] 3s2 3p1. The number of electrons in each element’s electron shells, particularly the outermost valence shell, is the primary factor in determining its chemical bonding behavior. But for most of the transition. In the periodic table, the elements. Aluminum Atom Have Valence Electrons.
From quizizz.com
Review Lesson Ionic Bonds and Naming Science Quizizz Aluminum Atom Have Valence Electrons Electron configuration of aluminium is [ne] 3s2 3p1. The number of electrons in each element’s electron shells, particularly the outermost valence shell, is the primary factor in determining its chemical bonding behavior. The outermost orbitals to be filled are 3s and 3p. 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom. Aluminum Atom Have Valence Electrons.
From galvinconanstuart.blogspot.com
Valence Electron Diagram General Wiring Diagram Aluminum Atom Have Valence Electrons What happens to aluminum when it reacts with. But for most of the transition. How many valence electrons are in one atom of each element? Write the configuration of aluminum, assuming that it has lost its valence electrons. An aluminum atom has three valence electrons. Helium (he) is located in group viiia (group 18). Do you think it will lose. Aluminum Atom Have Valence Electrons.
From www.nacimivo.me
structure électronique de l atome d aluminium structure électronique Aluminum Atom Have Valence Electrons Electron configuration of aluminium is [ne] 3s2 3p1. How many valence electrons are in one atom of each element? An aluminum atom has three valence electrons. Aluminum is the third element in the third row of the periodic table. The outermost orbitals to be filled are 3s and 3p. What happens to aluminum when it reacts with. Do you think. Aluminum Atom Have Valence Electrons.
From valenceelectrons.com
Protons, Neutrons, Electrons for Aluminum (Al, Al3+) Aluminum Atom Have Valence Electrons However, one atom only has two electrons, so it could never have more than 2 valence electrons. How many valence electrons are in one atom of each element? An aluminum atom has three valence electrons. 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or form—are those that. Helium. Aluminum Atom Have Valence Electrons.