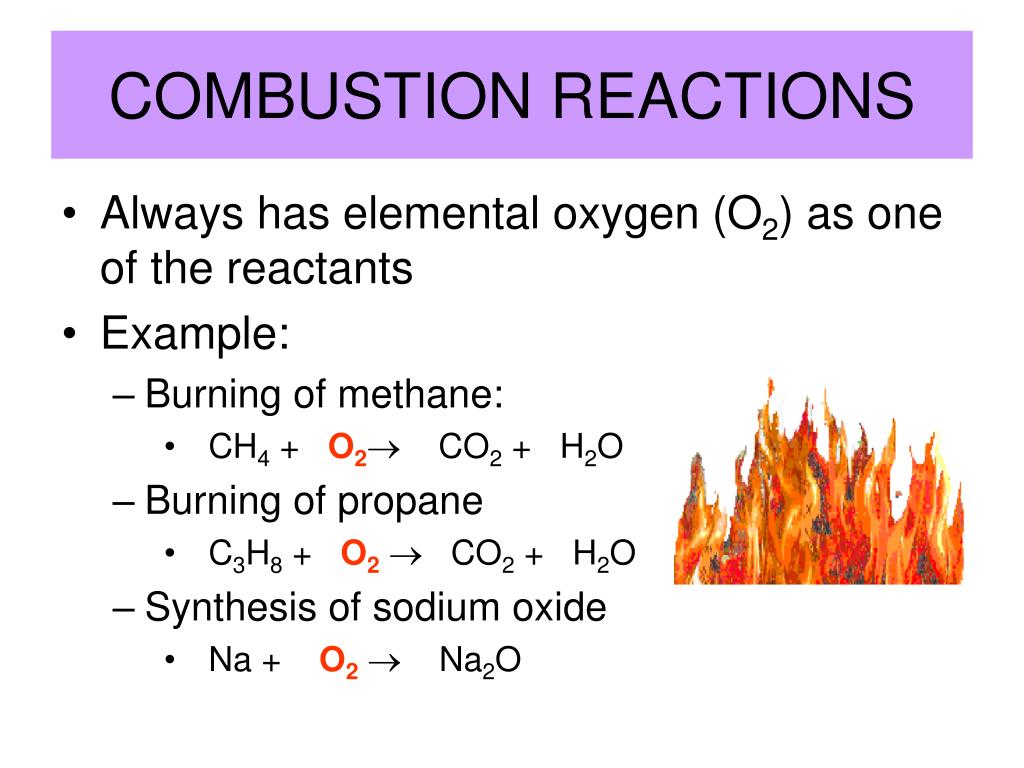
What Are The Products Of Most Combustion Reactions . Such reactions often liberate only water and carbon dioxide as. A familiar example of a combustion reaction is a lighted match. Combustion reactions must have oxygen as a reactant. Combustion requires three things to occur: When fuels burn in combustion reactions, they release useful thermal energy (heat). Combustion reactions are used to heat our homes, power most cars, and to generate a lot of our. Note that the water produced is in the gas state, rather than the liquid state, because. Complete combustion reactions, also known as clean combustion reactions, is the complete oxidation of the fuel. An initial ignition source, such as a match; Many combustion reactions occur with a hydrocarbon, a compound comprised solely of carbon and hydrogen. When a match is struck, friction heats the head to a temperature at which the chemicals react and generate.
from www.slideserve.com
When a match is struck, friction heats the head to a temperature at which the chemicals react and generate. Complete combustion reactions, also known as clean combustion reactions, is the complete oxidation of the fuel. Combustion reactions must have oxygen as a reactant. Combustion reactions are used to heat our homes, power most cars, and to generate a lot of our. Many combustion reactions occur with a hydrocarbon, a compound comprised solely of carbon and hydrogen. Note that the water produced is in the gas state, rather than the liquid state, because. Such reactions often liberate only water and carbon dioxide as. An initial ignition source, such as a match; When fuels burn in combustion reactions, they release useful thermal energy (heat). A familiar example of a combustion reaction is a lighted match.
PPT CHEMICAL REACTIONS AND EQUATIONS PowerPoint Presentation, free
What Are The Products Of Most Combustion Reactions Combustion reactions are used to heat our homes, power most cars, and to generate a lot of our. Note that the water produced is in the gas state, rather than the liquid state, because. When fuels burn in combustion reactions, they release useful thermal energy (heat). Many combustion reactions occur with a hydrocarbon, a compound comprised solely of carbon and hydrogen. When a match is struck, friction heats the head to a temperature at which the chemicals react and generate. Combustion requires three things to occur: An initial ignition source, such as a match; Such reactions often liberate only water and carbon dioxide as. A familiar example of a combustion reaction is a lighted match. Combustion reactions must have oxygen as a reactant. Combustion reactions are used to heat our homes, power most cars, and to generate a lot of our. Complete combustion reactions, also known as clean combustion reactions, is the complete oxidation of the fuel.
From www.slideserve.com
PPT Chemical Reactions Part 2 Combustion and Single Displacement What Are The Products Of Most Combustion Reactions Many combustion reactions occur with a hydrocarbon, a compound comprised solely of carbon and hydrogen. Such reactions often liberate only water and carbon dioxide as. Combustion requires three things to occur: When a match is struck, friction heats the head to a temperature at which the chemicals react and generate. An initial ignition source, such as a match; When fuels. What Are The Products Of Most Combustion Reactions.
From sciencenotes.org
Combustion Reaction Definition and Examples What Are The Products Of Most Combustion Reactions Note that the water produced is in the gas state, rather than the liquid state, because. Combustion requires three things to occur: Many combustion reactions occur with a hydrocarbon, a compound comprised solely of carbon and hydrogen. A familiar example of a combustion reaction is a lighted match. When fuels burn in combustion reactions, they release useful thermal energy (heat).. What Are The Products Of Most Combustion Reactions.
From www.chemistrylearner.com
Combustion Reaction Definition, Characteristics & Examples What Are The Products Of Most Combustion Reactions Note that the water produced is in the gas state, rather than the liquid state, because. Complete combustion reactions, also known as clean combustion reactions, is the complete oxidation of the fuel. Many combustion reactions occur with a hydrocarbon, a compound comprised solely of carbon and hydrogen. Such reactions often liberate only water and carbon dioxide as. Combustion reactions must. What Are The Products Of Most Combustion Reactions.
From www.thoughtco.com
What Is a Combustion Reaction? Definition and Examples What Are The Products Of Most Combustion Reactions Combustion reactions are used to heat our homes, power most cars, and to generate a lot of our. Many combustion reactions occur with a hydrocarbon, a compound comprised solely of carbon and hydrogen. When fuels burn in combustion reactions, they release useful thermal energy (heat). A familiar example of a combustion reaction is a lighted match. Complete combustion reactions, also. What Are The Products Of Most Combustion Reactions.
From sciencetrends.com
Combustion Reaction Examples And Definition Science Trends What Are The Products Of Most Combustion Reactions Note that the water produced is in the gas state, rather than the liquid state, because. Combustion reactions are used to heat our homes, power most cars, and to generate a lot of our. Many combustion reactions occur with a hydrocarbon, a compound comprised solely of carbon and hydrogen. Combustion requires three things to occur: Such reactions often liberate only. What Are The Products Of Most Combustion Reactions.
From www.chemicals.co.uk
Examples of Combustion Reactions in Chemistry The Chemistry Blog What Are The Products Of Most Combustion Reactions When fuels burn in combustion reactions, they release useful thermal energy (heat). Combustion reactions are used to heat our homes, power most cars, and to generate a lot of our. Note that the water produced is in the gas state, rather than the liquid state, because. Many combustion reactions occur with a hydrocarbon, a compound comprised solely of carbon and. What Are The Products Of Most Combustion Reactions.
From www.slideserve.com
PPT Combustion PowerPoint Presentation, free download ID1992427 What Are The Products Of Most Combustion Reactions A familiar example of a combustion reaction is a lighted match. Combustion reactions must have oxygen as a reactant. An initial ignition source, such as a match; When a match is struck, friction heats the head to a temperature at which the chemicals react and generate. Such reactions often liberate only water and carbon dioxide as. Combustion requires three things. What Are The Products Of Most Combustion Reactions.
From sciencetallis.weebly.com
7. Organic Chemistry THOMAS TALLIS SCIENCE What Are The Products Of Most Combustion Reactions When fuels burn in combustion reactions, they release useful thermal energy (heat). Combustion reactions are used to heat our homes, power most cars, and to generate a lot of our. Combustion requires three things to occur: Many combustion reactions occur with a hydrocarbon, a compound comprised solely of carbon and hydrogen. When a match is struck, friction heats the head. What Are The Products Of Most Combustion Reactions.
From ar.inspiredpencil.com
Combustion Reaction Diagram What Are The Products Of Most Combustion Reactions Combustion requires three things to occur: Combustion reactions must have oxygen as a reactant. When a match is struck, friction heats the head to a temperature at which the chemicals react and generate. When fuels burn in combustion reactions, they release useful thermal energy (heat). An initial ignition source, such as a match; Complete combustion reactions, also known as clean. What Are The Products Of Most Combustion Reactions.
From www.slideserve.com
PPT Combustion Reactions PowerPoint Presentation, free download ID What Are The Products Of Most Combustion Reactions An initial ignition source, such as a match; Combustion requires three things to occur: Such reactions often liberate only water and carbon dioxide as. Note that the water produced is in the gas state, rather than the liquid state, because. A familiar example of a combustion reaction is a lighted match. Combustion reactions are used to heat our homes, power. What Are The Products Of Most Combustion Reactions.
From www.slideserve.com
PPT Types of Chemical Reactions PowerPoint Presentation, free What Are The Products Of Most Combustion Reactions An initial ignition source, such as a match; Combustion reactions must have oxygen as a reactant. Complete combustion reactions, also known as clean combustion reactions, is the complete oxidation of the fuel. Note that the water produced is in the gas state, rather than the liquid state, because. Combustion reactions are used to heat our homes, power most cars, and. What Are The Products Of Most Combustion Reactions.
From www.grc.nasa.gov
Combustion What Are The Products Of Most Combustion Reactions Complete combustion reactions, also known as clean combustion reactions, is the complete oxidation of the fuel. Combustion requires three things to occur: Many combustion reactions occur with a hydrocarbon, a compound comprised solely of carbon and hydrogen. When fuels burn in combustion reactions, they release useful thermal energy (heat). Such reactions often liberate only water and carbon dioxide as. A. What Are The Products Of Most Combustion Reactions.
From www.youtube.com
Combustion Reactions YouTube What Are The Products Of Most Combustion Reactions Many combustion reactions occur with a hydrocarbon, a compound comprised solely of carbon and hydrogen. An initial ignition source, such as a match; Combustion reactions are used to heat our homes, power most cars, and to generate a lot of our. A familiar example of a combustion reaction is a lighted match. When fuels burn in combustion reactions, they release. What Are The Products Of Most Combustion Reactions.
From www.slideserve.com
PPT Chemical Reactions PowerPoint Presentation, free download ID What Are The Products Of Most Combustion Reactions Note that the water produced is in the gas state, rather than the liquid state, because. Combustion requires three things to occur: Such reactions often liberate only water and carbon dioxide as. Many combustion reactions occur with a hydrocarbon, a compound comprised solely of carbon and hydrogen. A familiar example of a combustion reaction is a lighted match. When a. What Are The Products Of Most Combustion Reactions.
From www.youtube.com
Balancing Combustion Reactions YouTube What Are The Products Of Most Combustion Reactions When a match is struck, friction heats the head to a temperature at which the chemicals react and generate. Note that the water produced is in the gas state, rather than the liquid state, because. Many combustion reactions occur with a hydrocarbon, a compound comprised solely of carbon and hydrogen. An initial ignition source, such as a match; Combustion reactions. What Are The Products Of Most Combustion Reactions.
From www.slideserve.com
PPT Chemical Reactions PowerPoint Presentation ID632515 What Are The Products Of Most Combustion Reactions Combustion reactions must have oxygen as a reactant. Many combustion reactions occur with a hydrocarbon, a compound comprised solely of carbon and hydrogen. Combustion requires three things to occur: An initial ignition source, such as a match; Note that the water produced is in the gas state, rather than the liquid state, because. Complete combustion reactions, also known as clean. What Are The Products Of Most Combustion Reactions.
From www.slideserve.com
PPT Combustion Reactions PowerPoint Presentation, free download ID What Are The Products Of Most Combustion Reactions Combustion requires three things to occur: Many combustion reactions occur with a hydrocarbon, a compound comprised solely of carbon and hydrogen. When fuels burn in combustion reactions, they release useful thermal energy (heat). A familiar example of a combustion reaction is a lighted match. Such reactions often liberate only water and carbon dioxide as. When a match is struck, friction. What Are The Products Of Most Combustion Reactions.
From www.slideserve.com
PPT Chapter 11 PowerPoint Presentation, free download ID6784177 What Are The Products Of Most Combustion Reactions Combustion reactions are used to heat our homes, power most cars, and to generate a lot of our. When a match is struck, friction heats the head to a temperature at which the chemicals react and generate. When fuels burn in combustion reactions, they release useful thermal energy (heat). Combustion reactions must have oxygen as a reactant. Combustion requires three. What Are The Products Of Most Combustion Reactions.
From www.slideserve.com
PPT Combustion PowerPoint Presentation, free download ID5525141 What Are The Products Of Most Combustion Reactions Combustion requires three things to occur: Combustion reactions must have oxygen as a reactant. When fuels burn in combustion reactions, they release useful thermal energy (heat). Many combustion reactions occur with a hydrocarbon, a compound comprised solely of carbon and hydrogen. Note that the water produced is in the gas state, rather than the liquid state, because. Such reactions often. What Are The Products Of Most Combustion Reactions.
From www.slideserve.com
PPT CHEMICAL REACTIONS AND EQUATIONS PowerPoint Presentation, free What Are The Products Of Most Combustion Reactions When fuels burn in combustion reactions, they release useful thermal energy (heat). A familiar example of a combustion reaction is a lighted match. Such reactions often liberate only water and carbon dioxide as. Combustion reactions are used to heat our homes, power most cars, and to generate a lot of our. An initial ignition source, such as a match; Note. What Are The Products Of Most Combustion Reactions.
From www.shalom-education.com
Fuels and Combustion KS3 Chemistry Revision What Are The Products Of Most Combustion Reactions Note that the water produced is in the gas state, rather than the liquid state, because. Combustion reactions are used to heat our homes, power most cars, and to generate a lot of our. When fuels burn in combustion reactions, they release useful thermal energy (heat). Combustion requires three things to occur: Combustion reactions must have oxygen as a reactant.. What Are The Products Of Most Combustion Reactions.
From www.slideserve.com
PPT COMBUSTION REACTIONS PowerPoint Presentation, free download ID What Are The Products Of Most Combustion Reactions Many combustion reactions occur with a hydrocarbon, a compound comprised solely of carbon and hydrogen. Such reactions often liberate only water and carbon dioxide as. Note that the water produced is in the gas state, rather than the liquid state, because. Combustion requires three things to occur: When fuels burn in combustion reactions, they release useful thermal energy (heat). Combustion. What Are The Products Of Most Combustion Reactions.
From www.slideserve.com
PPT COMBUSTION REACTIONS PowerPoint Presentation, free download ID What Are The Products Of Most Combustion Reactions A familiar example of a combustion reaction is a lighted match. Combustion reactions must have oxygen as a reactant. Complete combustion reactions, also known as clean combustion reactions, is the complete oxidation of the fuel. An initial ignition source, such as a match; Such reactions often liberate only water and carbon dioxide as. Combustion reactions are used to heat our. What Are The Products Of Most Combustion Reactions.
From study.com
Predicting the Products of a Combustion Reaction Chemistry What Are The Products Of Most Combustion Reactions When fuels burn in combustion reactions, they release useful thermal energy (heat). Note that the water produced is in the gas state, rather than the liquid state, because. Complete combustion reactions, also known as clean combustion reactions, is the complete oxidation of the fuel. An initial ignition source, such as a match; When a match is struck, friction heats the. What Are The Products Of Most Combustion Reactions.
From wou.edu
CH150 Chapter 5 Chemical Reactions Chemistry What Are The Products Of Most Combustion Reactions Combustion requires three things to occur: Combustion reactions must have oxygen as a reactant. When fuels burn in combustion reactions, they release useful thermal energy (heat). When a match is struck, friction heats the head to a temperature at which the chemicals react and generate. Complete combustion reactions, also known as clean combustion reactions, is the complete oxidation of the. What Are The Products Of Most Combustion Reactions.
From slidetodoc.com
Combustion Reactions Combustion is a chemical reaction where What Are The Products Of Most Combustion Reactions When a match is struck, friction heats the head to a temperature at which the chemicals react and generate. Note that the water produced is in the gas state, rather than the liquid state, because. Such reactions often liberate only water and carbon dioxide as. Complete combustion reactions, also known as clean combustion reactions, is the complete oxidation of the. What Are The Products Of Most Combustion Reactions.
From www.slideserve.com
PPT Chapter 6 Chemical Reactions PowerPoint Presentation, free What Are The Products Of Most Combustion Reactions An initial ignition source, such as a match; Complete combustion reactions, also known as clean combustion reactions, is the complete oxidation of the fuel. Many combustion reactions occur with a hydrocarbon, a compound comprised solely of carbon and hydrogen. Combustion reactions must have oxygen as a reactant. A familiar example of a combustion reaction is a lighted match. When a. What Are The Products Of Most Combustion Reactions.
From www.slideserve.com
PPT Combustion Reactions PowerPoint Presentation, free download ID What Are The Products Of Most Combustion Reactions Combustion requires three things to occur: Combustion reactions are used to heat our homes, power most cars, and to generate a lot of our. Such reactions often liberate only water and carbon dioxide as. An initial ignition source, such as a match; Many combustion reactions occur with a hydrocarbon, a compound comprised solely of carbon and hydrogen. Complete combustion reactions,. What Are The Products Of Most Combustion Reactions.
From www.slideserve.com
PPT Types of Chemical Reactions PowerPoint Presentation, free What Are The Products Of Most Combustion Reactions A familiar example of a combustion reaction is a lighted match. Note that the water produced is in the gas state, rather than the liquid state, because. Complete combustion reactions, also known as clean combustion reactions, is the complete oxidation of the fuel. Many combustion reactions occur with a hydrocarbon, a compound comprised solely of carbon and hydrogen. Such reactions. What Are The Products Of Most Combustion Reactions.
From in.pinterest.com
Types of Chemical Reactions Chemical reactions, Chemistry basics What Are The Products Of Most Combustion Reactions When fuels burn in combustion reactions, they release useful thermal energy (heat). Combustion reactions are used to heat our homes, power most cars, and to generate a lot of our. Many combustion reactions occur with a hydrocarbon, a compound comprised solely of carbon and hydrogen. Such reactions often liberate only water and carbon dioxide as. Combustion reactions must have oxygen. What Are The Products Of Most Combustion Reactions.
From www.youtube.com
Chemical Reactions (3 of 11) Combustion Reactions, An Explanation YouTube What Are The Products Of Most Combustion Reactions When fuels burn in combustion reactions, they release useful thermal energy (heat). Many combustion reactions occur with a hydrocarbon, a compound comprised solely of carbon and hydrogen. An initial ignition source, such as a match; Combustion requires three things to occur: Combustion reactions are used to heat our homes, power most cars, and to generate a lot of our. Complete. What Are The Products Of Most Combustion Reactions.
From treatybottle13.pythonanywhere.com
Simple Combustion Reaction Octane Physics Formulas Of Class 12 What Are The Products Of Most Combustion Reactions When fuels burn in combustion reactions, they release useful thermal energy (heat). Such reactions often liberate only water and carbon dioxide as. Combustion reactions must have oxygen as a reactant. Note that the water produced is in the gas state, rather than the liquid state, because. An initial ignition source, such as a match; A familiar example of a combustion. What Are The Products Of Most Combustion Reactions.
From www.sciencephoto.com
Combustion reaction, illustration Stock Image F027/1851 Science What Are The Products Of Most Combustion Reactions Combustion reactions must have oxygen as a reactant. Combustion requires three things to occur: An initial ignition source, such as a match; When a match is struck, friction heats the head to a temperature at which the chemicals react and generate. When fuels burn in combustion reactions, they release useful thermal energy (heat). Complete combustion reactions, also known as clean. What Are The Products Of Most Combustion Reactions.
From www.tes.com
Secondary chemistry teaching resources Chemical reactions TES What Are The Products Of Most Combustion Reactions Such reactions often liberate only water and carbon dioxide as. Combustion reactions must have oxygen as a reactant. Complete combustion reactions, also known as clean combustion reactions, is the complete oxidation of the fuel. Note that the water produced is in the gas state, rather than the liquid state, because. Many combustion reactions occur with a hydrocarbon, a compound comprised. What Are The Products Of Most Combustion Reactions.
From www.slideserve.com
PPT Chapter 10 Chemical Reactions PowerPoint Presentation, free What Are The Products Of Most Combustion Reactions An initial ignition source, such as a match; Combustion reactions are used to heat our homes, power most cars, and to generate a lot of our. A familiar example of a combustion reaction is a lighted match. Combustion reactions must have oxygen as a reactant. Note that the water produced is in the gas state, rather than the liquid state,. What Are The Products Of Most Combustion Reactions.