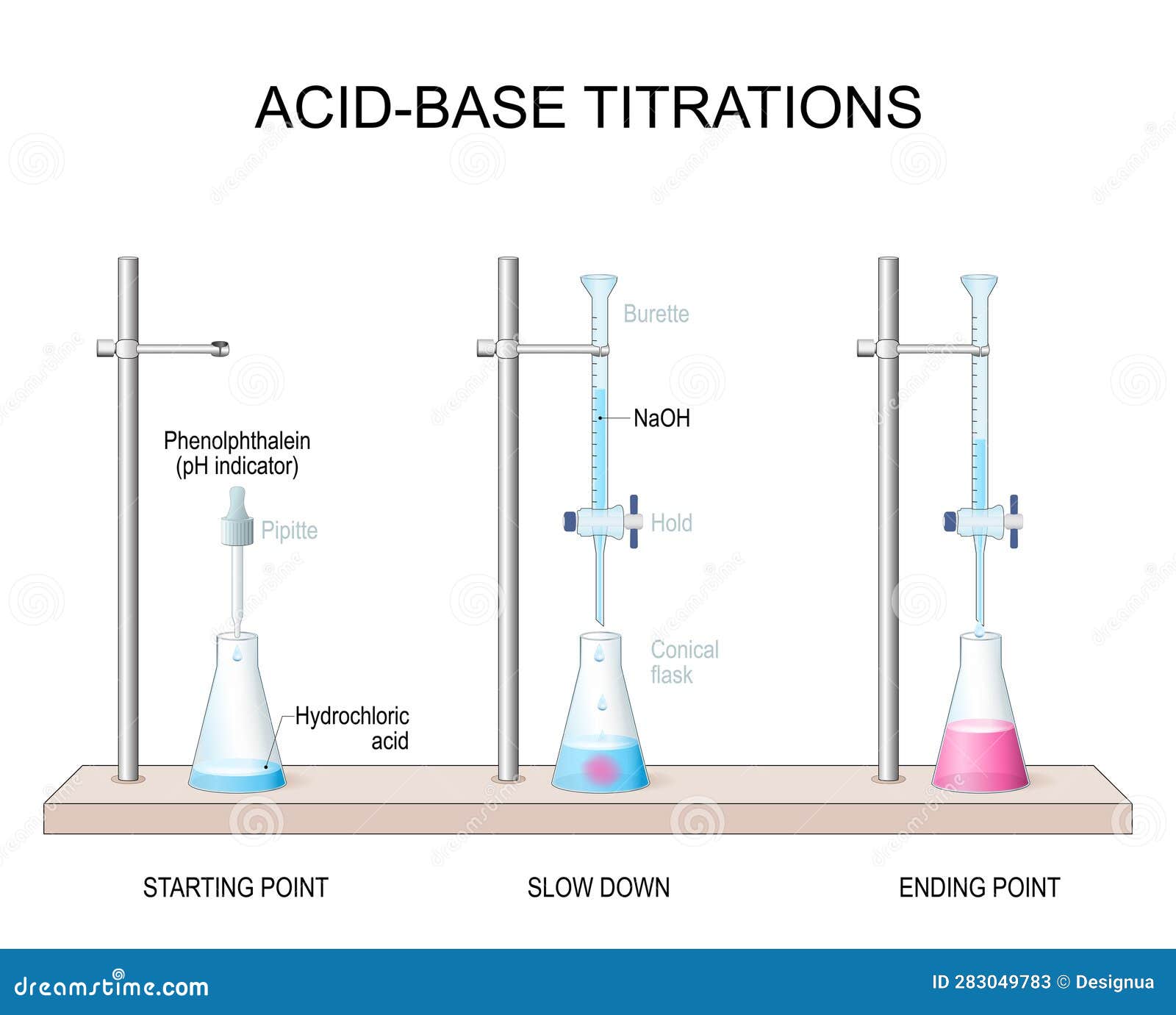
Apparatus Titration . titration is a way of measuring the concentration of something, usually the concentration of a substance in a solution. The reagent (titrant) is the solution with a known molarity that will react with the analyte. titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another. 1.1 a titration involves measuring the exact volume of a reagent solution (the titrant) that is required to react completely with. The analyte (titrand) is the solution with an unknown molarity. This resource accompanies the infographic poster and fact sheet titration apparatus. in an indicator based titration you add another chemical that changes color at the ph equal to the equivalence point,. titration apparatus teacher notes. For example, you can use.
from www.dreamstime.com
1.1 a titration involves measuring the exact volume of a reagent solution (the titrant) that is required to react completely with. titration apparatus teacher notes. The reagent (titrant) is the solution with a known molarity that will react with the analyte. in an indicator based titration you add another chemical that changes color at the ph equal to the equivalence point,. For example, you can use. The analyte (titrand) is the solution with an unknown molarity. This resource accompanies the infographic poster and fact sheet titration apparatus. titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another. titration is a way of measuring the concentration of something, usually the concentration of a substance in a solution.
Phenolphthalein Indicator in Acidbase Titration Stock Vector
Apparatus Titration This resource accompanies the infographic poster and fact sheet titration apparatus. This resource accompanies the infographic poster and fact sheet titration apparatus. titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another. titration is a way of measuring the concentration of something, usually the concentration of a substance in a solution. For example, you can use. The analyte (titrand) is the solution with an unknown molarity. in an indicator based titration you add another chemical that changes color at the ph equal to the equivalence point,. 1.1 a titration involves measuring the exact volume of a reagent solution (the titrant) that is required to react completely with. The reagent (titrant) is the solution with a known molarity that will react with the analyte. titration apparatus teacher notes.
From theedge.com.hk
Chemistry How To Titration The Edge Apparatus Titration 1.1 a titration involves measuring the exact volume of a reagent solution (the titrant) that is required to react completely with. This resource accompanies the infographic poster and fact sheet titration apparatus. The reagent (titrant) is the solution with a known molarity that will react with the analyte. The analyte (titrand) is the solution with an unknown molarity. . Apparatus Titration.
From gioajmlyz.blob.core.windows.net
Titration Chemistry Research at Nicole Apple blog Apparatus Titration The reagent (titrant) is the solution with a known molarity that will react with the analyte. 1.1 a titration involves measuring the exact volume of a reagent solution (the titrant) that is required to react completely with. in an indicator based titration you add another chemical that changes color at the ph equal to the equivalence point,. The. Apparatus Titration.
From exymjkekq.blob.core.windows.net
Titration In Chemistry Equipment at Barbara Adamson blog Apparatus Titration This resource accompanies the infographic poster and fact sheet titration apparatus. The reagent (titrant) is the solution with a known molarity that will react with the analyte. in an indicator based titration you add another chemical that changes color at the ph equal to the equivalence point,. titration is a way of measuring the concentration of something, usually. Apparatus Titration.
From www.vrogue.co
Acid Base Titration Volumetric Analysis Slides Pdf Ac vrogue.co Apparatus Titration For example, you can use. titration is a way of measuring the concentration of something, usually the concentration of a substance in a solution. 1.1 a titration involves measuring the exact volume of a reagent solution (the titrant) that is required to react completely with. The reagent (titrant) is the solution with a known molarity that will react. Apparatus Titration.
From www.chemicals.co.uk
Titration Experiments In Chemistry The Chemistry Blog Apparatus Titration This resource accompanies the infographic poster and fact sheet titration apparatus. The reagent (titrant) is the solution with a known molarity that will react with the analyte. titration apparatus teacher notes. The analyte (titrand) is the solution with an unknown molarity. titration is a way of measuring the concentration of something, usually the concentration of a substance in. Apparatus Titration.
From exovgoxgf.blob.core.windows.net
Acid Lab Equipment Upgrade at Michele Eubank blog Apparatus Titration This resource accompanies the infographic poster and fact sheet titration apparatus. titration is a way of measuring the concentration of something, usually the concentration of a substance in a solution. in an indicator based titration you add another chemical that changes color at the ph equal to the equivalence point,. titration is the slow addition of one. Apparatus Titration.
From chemistrylabs-2.blogspot.com
Titration Apparatus Diagram Chemistry Labs Apparatus Titration titration apparatus teacher notes. titration is a way of measuring the concentration of something, usually the concentration of a substance in a solution. 1.1 a titration involves measuring the exact volume of a reagent solution (the titrant) that is required to react completely with. For example, you can use. The reagent (titrant) is the solution with a. Apparatus Titration.
From theory.labster.com
TitrationsApparat Labster Theory Apparatus Titration The analyte (titrand) is the solution with an unknown molarity. titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another. 1.1 a titration involves measuring the exact volume of a reagent solution (the titrant) that is required to react completely with. titration is a way of. Apparatus Titration.
From pharmaceuticaleducation.blogspot.com
Pharma gyan Pandit Apparatus Titration titration is a way of measuring the concentration of something, usually the concentration of a substance in a solution. This resource accompanies the infographic poster and fact sheet titration apparatus. The reagent (titrant) is the solution with a known molarity that will react with the analyte. titration apparatus teacher notes. in an indicator based titration you add. Apparatus Titration.
From www.vecteezy.com
Acid base titration experiment and phases of color change during Apparatus Titration titration apparatus teacher notes. For example, you can use. in an indicator based titration you add another chemical that changes color at the ph equal to the equivalence point,. The reagent (titrant) is the solution with a known molarity that will react with the analyte. titration is the slow addition of one solution of a known concentration. Apparatus Titration.
From www.labfriend.com.au
Titration apparatus, BLAUBRAND, class AS, n. pellet, without stopcock Apparatus Titration titration is a way of measuring the concentration of something, usually the concentration of a substance in a solution. titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another. The analyte (titrand) is the solution with an unknown molarity. titration apparatus teacher notes. The reagent (titrant). Apparatus Titration.
From ar.inspiredpencil.com
Titration Setup Diagram Apparatus Titration The analyte (titrand) is the solution with an unknown molarity. in an indicator based titration you add another chemical that changes color at the ph equal to the equivalence point,. 1.1 a titration involves measuring the exact volume of a reagent solution (the titrant) that is required to react completely with. For example, you can use. titration. Apparatus Titration.
From cartoondealer.com
Redox Titration With Burette And Pipette Cartoon Vector CartoonDealer Apparatus Titration For example, you can use. titration is a way of measuring the concentration of something, usually the concentration of a substance in a solution. The reagent (titrant) is the solution with a known molarity that will react with the analyte. in an indicator based titration you add another chemical that changes color at the ph equal to the. Apparatus Titration.
From www.labfriend.com.au
Titration apparatus 251/10 without bottle LabFriend Australia Apparatus Titration titration apparatus teacher notes. This resource accompanies the infographic poster and fact sheet titration apparatus. titration is a way of measuring the concentration of something, usually the concentration of a substance in a solution. in an indicator based titration you add another chemical that changes color at the ph equal to the equivalence point,. titration is. Apparatus Titration.
From www.dreamstime.com
Phenolphthalein Indicator in Acidbase Titration Stock Vector Apparatus Titration titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another. The reagent (titrant) is the solution with a known molarity that will react with the analyte. The analyte (titrand) is the solution with an unknown molarity. This resource accompanies the infographic poster and fact sheet titration apparatus. . Apparatus Titration.
From www.homesciencetools.com
Titration Kit Titration Lab Equipment for Chemistry HST Apparatus Titration titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another. titration apparatus teacher notes. The analyte (titrand) is the solution with an unknown molarity. For example, you can use. 1.1 a titration involves measuring the exact volume of a reagent solution (the titrant) that is required. Apparatus Titration.
From classnotes.org.in
Acid Base Titration using Indicator Chemistry, Class 11, Ionic Apparatus Titration titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another. titration is a way of measuring the concentration of something, usually the concentration of a substance in a solution. This resource accompanies the infographic poster and fact sheet titration apparatus. 1.1 a titration involves measuring the. Apparatus Titration.
From www.mhenterprises.net
Titration Apparatus Melting Point Apparatus Manufacturer from Hyderabad Apparatus Titration titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another. The analyte (titrand) is the solution with an unknown molarity. This resource accompanies the infographic poster and fact sheet titration apparatus. titration is a way of measuring the concentration of something, usually the concentration of a substance. Apparatus Titration.
From mavink.com
Titration Apparatus Diagram Apparatus Titration 1.1 a titration involves measuring the exact volume of a reagent solution (the titrant) that is required to react completely with. The analyte (titrand) is the solution with an unknown molarity. in an indicator based titration you add another chemical that changes color at the ph equal to the equivalence point,. For example, you can use. titration. Apparatus Titration.
From giojgvkot.blob.core.windows.net
Titration For Dummies at Melissa Espada blog Apparatus Titration The reagent (titrant) is the solution with a known molarity that will react with the analyte. The analyte (titrand) is the solution with an unknown molarity. in an indicator based titration you add another chemical that changes color at the ph equal to the equivalence point,. 1.1 a titration involves measuring the exact volume of a reagent solution. Apparatus Titration.
From chem.libretexts.org
11 Titration of Vinegar (Experiment) Chemistry LibreTexts Apparatus Titration titration apparatus teacher notes. The reagent (titrant) is the solution with a known molarity that will react with the analyte. 1.1 a titration involves measuring the exact volume of a reagent solution (the titrant) that is required to react completely with. titration is a way of measuring the concentration of something, usually the concentration of a substance. Apparatus Titration.
From acechemistry.co.uk
Preparing apparatus for a titration advanced Ace Chemistry Apparatus Titration titration is a way of measuring the concentration of something, usually the concentration of a substance in a solution. 1.1 a titration involves measuring the exact volume of a reagent solution (the titrant) that is required to react completely with. The analyte (titrand) is the solution with an unknown molarity. This resource accompanies the infographic poster and fact. Apparatus Titration.
From fyoioeyql.blob.core.windows.net
Titration Applications In Industry at Robert Carson blog Apparatus Titration in an indicator based titration you add another chemical that changes color at the ph equal to the equivalence point,. titration is a way of measuring the concentration of something, usually the concentration of a substance in a solution. The reagent (titrant) is the solution with a known molarity that will react with the analyte. titration apparatus. Apparatus Titration.
From www.hoddereducationmagazines.com
Performing the perfect titration Hodder Education Magazines Apparatus Titration The analyte (titrand) is the solution with an unknown molarity. titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another. titration apparatus teacher notes. in an indicator based titration you add another chemical that changes color at the ph equal to the equivalence point,. 1.1. Apparatus Titration.
From springofchemistry.blogspot.com
Spring Of Chemistry Diagram for titration Apparatus Titration titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another. in an indicator based titration you add another chemical that changes color at the ph equal to the equivalence point,. titration is a way of measuring the concentration of something, usually the concentration of a substance. Apparatus Titration.
From resource.studiaacademy.com
edexcel_igcse_chemistry_topic15_acidsalkalisandtitrations_004 Apparatus Titration titration apparatus teacher notes. For example, you can use. The analyte (titrand) is the solution with an unknown molarity. This resource accompanies the infographic poster and fact sheet titration apparatus. titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another. in an indicator based titration you. Apparatus Titration.
From nanbei-china.en.made-in-china.com
Economical Lab Automatic Potentiometric Titration Apparatus Potential Apparatus Titration For example, you can use. 1.1 a titration involves measuring the exact volume of a reagent solution (the titrant) that is required to react completely with. titration is a way of measuring the concentration of something, usually the concentration of a substance in a solution. in an indicator based titration you add another chemical that changes color. Apparatus Titration.
From www.alamy.com
Titration apparatus at chemical laboratory Stock Photo 29817654 Alamy Apparatus Titration This resource accompanies the infographic poster and fact sheet titration apparatus. titration is a way of measuring the concentration of something, usually the concentration of a substance in a solution. The reagent (titrant) is the solution with a known molarity that will react with the analyte. The analyte (titrand) is the solution with an unknown molarity. titration is. Apparatus Titration.
From iconspng.com
Titration Apparatus Icons PNG Free PNG and Icons Downloads Apparatus Titration The analyte (titrand) is the solution with an unknown molarity. in an indicator based titration you add another chemical that changes color at the ph equal to the equivalence point,. titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another. titration is a way of measuring. Apparatus Titration.
From edu.rsc.org
Vintage titrations sulfur dioxide concentrations in wine Resource Apparatus Titration This resource accompanies the infographic poster and fact sheet titration apparatus. in an indicator based titration you add another chemical that changes color at the ph equal to the equivalence point,. 1.1 a titration involves measuring the exact volume of a reagent solution (the titrant) that is required to react completely with. The reagent (titrant) is the solution. Apparatus Titration.
From shop.fleischhacker.biz
Titrators, class AS, with intermediate stopcock, lateral stopcock with Apparatus Titration titration apparatus teacher notes. 1.1 a titration involves measuring the exact volume of a reagent solution (the titrant) that is required to react completely with. For example, you can use. titration is a way of measuring the concentration of something, usually the concentration of a substance in a solution. The analyte (titrand) is the solution with an. Apparatus Titration.
From www.savemyexams.com
Required Practical Strong Acid & Strong Alkali Titration AQA GCSE Apparatus Titration The analyte (titrand) is the solution with an unknown molarity. The reagent (titrant) is the solution with a known molarity that will react with the analyte. titration is a way of measuring the concentration of something, usually the concentration of a substance in a solution. titration is the slow addition of one solution of a known concentration (called. Apparatus Titration.
From www.chemistryscl.com
Titrimetry, Titration Classifications, Standard solutions, Equivalence Apparatus Titration titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another. titration is a way of measuring the concentration of something, usually the concentration of a substance in a solution. The reagent (titrant) is the solution with a known molarity that will react with the analyte. in. Apparatus Titration.
From gioajmlyz.blob.core.windows.net
Titration Chemistry Research at Nicole Apple blog Apparatus Titration 1.1 a titration involves measuring the exact volume of a reagent solution (the titrant) that is required to react completely with. titration is a way of measuring the concentration of something, usually the concentration of a substance in a solution. This resource accompanies the infographic poster and fact sheet titration apparatus. The analyte (titrand) is the solution with. Apparatus Titration.
From www.shalom-education.com
Required Practical Titration with a Strong Acid and a Strong Alkali Apparatus Titration The analyte (titrand) is the solution with an unknown molarity. titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another. titration is a way of measuring the concentration of something, usually the concentration of a substance in a solution. For example, you can use. 1.1 a. Apparatus Titration.