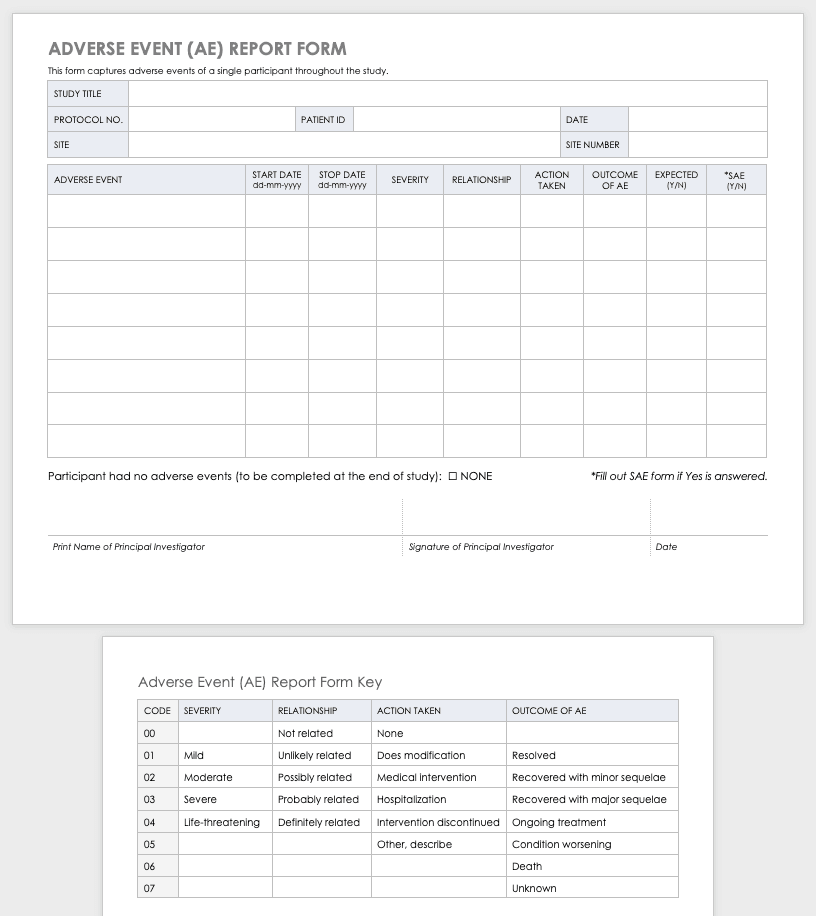
Case Report Form Clinical Trial . 1.2 the case report form (crf) is a data collection tool used to capture the required data, as defined by the protocol, for each. The crf is used by the study sponsor to capture. a case report form (crf) is designed to collect the patient data in a clinical trial; what is a case report form? a case report form (crf) is a critical component of clinical trials and research studies. case report form (crf) is a specialized document in clinical research. It's a structured document used. the case report form (crf) is a pivotal tool in clinical research. A crf is a set of documents that collects data and information from a clinical trial. It is a document used in clinical trials to collect data from each. It should be study protocol driven, robust in content.
from www.sampletemplate.my.id
the case report form (crf) is a pivotal tool in clinical research. case report form (crf) is a specialized document in clinical research. 1.2 the case report form (crf) is a data collection tool used to capture the required data, as defined by the protocol, for each. It is a document used in clinical trials to collect data from each. what is a case report form? a case report form (crf) is a critical component of clinical trials and research studies. It's a structured document used. a case report form (crf) is designed to collect the patient data in a clinical trial; It should be study protocol driven, robust in content. The crf is used by the study sponsor to capture.
Monitoring Report Template Clinical Trials Sampletemplate.my.id
Case Report Form Clinical Trial It should be study protocol driven, robust in content. It is a document used in clinical trials to collect data from each. It's a structured document used. the case report form (crf) is a pivotal tool in clinical research. case report form (crf) is a specialized document in clinical research. 1.2 the case report form (crf) is a data collection tool used to capture the required data, as defined by the protocol, for each. The crf is used by the study sponsor to capture. a case report form (crf) is a critical component of clinical trials and research studies. It should be study protocol driven, robust in content. what is a case report form? A crf is a set of documents that collects data and information from a clinical trial. a case report form (crf) is designed to collect the patient data in a clinical trial;
From businesstemplateinspiration.blogspot.com
Case Report Form Template Clinical Trials Case Report Form Clinical Trial a case report form (crf) is a critical component of clinical trials and research studies. A crf is a set of documents that collects data and information from a clinical trial. The crf is used by the study sponsor to capture. case report form (crf) is a specialized document in clinical research. It is a document used in. Case Report Form Clinical Trial.
From www.pinterest.com
Clinical Trial Report Template (3) TEMPLATES EXAMPLE TEMPLATES Case Report Form Clinical Trial case report form (crf) is a specialized document in clinical research. a case report form (crf) is a critical component of clinical trials and research studies. A crf is a set of documents that collects data and information from a clinical trial. a case report form (crf) is designed to collect the patient data in a clinical. Case Report Form Clinical Trial.
From designtemplatework.blogspot.com
Case Report Form Template Clinical Trials Free Design Template for Case Report Form Clinical Trial It's a structured document used. A crf is a set of documents that collects data and information from a clinical trial. a case report form (crf) is a critical component of clinical trials and research studies. It is a document used in clinical trials to collect data from each. what is a case report form? The crf is. Case Report Form Clinical Trial.
From www.altexsoft.com
Clinical Trial Software EDC, CTMS, ePRO, RTSM AltexSoft Case Report Form Clinical Trial case report form (crf) is a specialized document in clinical research. It's a structured document used. a case report form (crf) is designed to collect the patient data in a clinical trial; It should be study protocol driven, robust in content. It is a document used in clinical trials to collect data from each. 1.2 the case. Case Report Form Clinical Trial.
From www.xfanzexpo.com
Free Clinical Trial Templates Smartsheet in Case Report Form Template Case Report Form Clinical Trial what is a case report form? case report form (crf) is a specialized document in clinical research. 1.2 the case report form (crf) is a data collection tool used to capture the required data, as defined by the protocol, for each. a case report form (crf) is designed to collect the patient data in a clinical. Case Report Form Clinical Trial.
From www.youtube.com
Case Report Form in Clinical Research YouTube Case Report Form Clinical Trial a case report form (crf) is a critical component of clinical trials and research studies. A crf is a set of documents that collects data and information from a clinical trial. the case report form (crf) is a pivotal tool in clinical research. a case report form (crf) is designed to collect the patient data in a. Case Report Form Clinical Trial.
From www.xfanzexpo.com
Case Report Form Template Clinical Trials Large Study for Trial Report Case Report Form Clinical Trial It's a structured document used. It should be study protocol driven, robust in content. a case report form (crf) is a critical component of clinical trials and research studies. A crf is a set of documents that collects data and information from a clinical trial. The crf is used by the study sponsor to capture. 1.2 the case. Case Report Form Clinical Trial.
From www.sampleforms.com
FREE 15+ Case Report Forms in PDF MS Word Case Report Form Clinical Trial It's a structured document used. a case report form (crf) is a critical component of clinical trials and research studies. It is a document used in clinical trials to collect data from each. what is a case report form? 1.2 the case report form (crf) is a data collection tool used to capture the required data, as. Case Report Form Clinical Trial.
From businesstemplateinspiration.blogspot.com
Case Report Form Template Clinical Trials Case Report Form Clinical Trial 1.2 the case report form (crf) is a data collection tool used to capture the required data, as defined by the protocol, for each. It is a document used in clinical trials to collect data from each. It's a structured document used. A crf is a set of documents that collects data and information from a clinical trial. . Case Report Form Clinical Trial.
From bestprofessionaltemplate.blogspot.com
Clinical Trial Report Template Case Report Form Clinical Trial the case report form (crf) is a pivotal tool in clinical research. It should be study protocol driven, robust in content. 1.2 the case report form (crf) is a data collection tool used to capture the required data, as defined by the protocol, for each. a case report form (crf) is a critical component of clinical trials. Case Report Form Clinical Trial.
From newcreativetemplateideas.blogspot.com
Case Report Form Template Clinical Trials New Creative Template Ideas Case Report Form Clinical Trial The crf is used by the study sponsor to capture. A crf is a set of documents that collects data and information from a clinical trial. It should be study protocol driven, robust in content. 1.2 the case report form (crf) is a data collection tool used to capture the required data, as defined by the protocol, for each.. Case Report Form Clinical Trial.
From www.sampletemplate.my.id
Monitoring Report Template Clinical Trials Sampletemplate.my.id Case Report Form Clinical Trial a case report form (crf) is designed to collect the patient data in a clinical trial; the case report form (crf) is a pivotal tool in clinical research. what is a case report form? It should be study protocol driven, robust in content. 1.2 the case report form (crf) is a data collection tool used to. Case Report Form Clinical Trial.
From www.atlanticcityaquarium.com
Case Report Form Template Clinical Trials Case Report Form Clinical Trial It should be study protocol driven, robust in content. the case report form (crf) is a pivotal tool in clinical research. 1.2 the case report form (crf) is a data collection tool used to capture the required data, as defined by the protocol, for each. The crf is used by the study sponsor to capture. It's a structured. Case Report Form Clinical Trial.
From www.cumed.org
Case Eport Form Electronic Qolty Format Ppt In Clinical for Clinical Case Report Form Clinical Trial case report form (crf) is a specialized document in clinical research. 1.2 the case report form (crf) is a data collection tool used to capture the required data, as defined by the protocol, for each. a case report form (crf) is a critical component of clinical trials and research studies. It should be study protocol driven, robust. Case Report Form Clinical Trial.
From www.sampleforms.com
FREE 15+ Case Report Forms in PDF MS Word Case Report Form Clinical Trial It is a document used in clinical trials to collect data from each. the case report form (crf) is a pivotal tool in clinical research. a case report form (crf) is designed to collect the patient data in a clinical trial; what is a case report form? a case report form (crf) is a critical component. Case Report Form Clinical Trial.
From www.sampleforms.com
What Is a Case Report Form? [ Importance, Tips, Samples ] Case Report Form Clinical Trial The crf is used by the study sponsor to capture. It is a document used in clinical trials to collect data from each. case report form (crf) is a specialized document in clinical research. a case report form (crf) is a critical component of clinical trials and research studies. A crf is a set of documents that collects. Case Report Form Clinical Trial.
From businessdesignlayouttemplates.blogspot.com
Case Report Form Template Business Design Layout Templates Case Report Form Clinical Trial It's a structured document used. the case report form (crf) is a pivotal tool in clinical research. 1.2 the case report form (crf) is a data collection tool used to capture the required data, as defined by the protocol, for each. It is a document used in clinical trials to collect data from each. The crf is used. Case Report Form Clinical Trial.
From restoringtrials.org
Blank Case Report Form RIAT Support Center Case Report Form Clinical Trial It should be study protocol driven, robust in content. what is a case report form? a case report form (crf) is designed to collect the patient data in a clinical trial; 1.2 the case report form (crf) is a data collection tool used to capture the required data, as defined by the protocol, for each. a. Case Report Form Clinical Trial.
From rollerworksfamilyskating.com
Case Report form Template Clinical Trials Case Report Form Clinical Trial The crf is used by the study sponsor to capture. 1.2 the case report form (crf) is a data collection tool used to capture the required data, as defined by the protocol, for each. a case report form (crf) is designed to collect the patient data in a clinical trial; It is a document used in clinical trials. Case Report Form Clinical Trial.
From www.atlanticcityaquarium.com
Case Report Form Template Clinical Trials Case Report Form Clinical Trial A crf is a set of documents that collects data and information from a clinical trial. It's a structured document used. The crf is used by the study sponsor to capture. a case report form (crf) is a critical component of clinical trials and research studies. 1.2 the case report form (crf) is a data collection tool used. Case Report Form Clinical Trial.
From pray.gelorailmu.com
Free 15+ Case Report Forms In Pdf Ms Word pertaining to Case Report Case Report Form Clinical Trial a case report form (crf) is designed to collect the patient data in a clinical trial; It is a document used in clinical trials to collect data from each. a case report form (crf) is a critical component of clinical trials and research studies. A crf is a set of documents that collects data and information from a. Case Report Form Clinical Trial.
From www.atlanticcityaquarium.com
Case Report Form Template Case Report Form Clinical Trial 1.2 the case report form (crf) is a data collection tool used to capture the required data, as defined by the protocol, for each. a case report form (crf) is a critical component of clinical trials and research studies. It's a structured document used. case report form (crf) is a specialized document in clinical research. A crf. Case Report Form Clinical Trial.
From studylib.net
Clinical Trial Pregnancy Form Case Report Form Clinical Trial a case report form (crf) is a critical component of clinical trials and research studies. 1.2 the case report form (crf) is a data collection tool used to capture the required data, as defined by the protocol, for each. It should be study protocol driven, robust in content. It's a structured document used. A crf is a set. Case Report Form Clinical Trial.
From www.rebeccachulew.com
Clinical Trial Report Template Case Report Form Clinical Trial The crf is used by the study sponsor to capture. It is a document used in clinical trials to collect data from each. a case report form (crf) is a critical component of clinical trials and research studies. It should be study protocol driven, robust in content. what is a case report form? 1.2 the case report. Case Report Form Clinical Trial.
From pray.gelorailmu.com
Free 15+ Case Report Forms In Pdf Ms Word inside Case Report Form Case Report Form Clinical Trial It is a document used in clinical trials to collect data from each. The crf is used by the study sponsor to capture. a case report form (crf) is designed to collect the patient data in a clinical trial; the case report form (crf) is a pivotal tool in clinical research. It should be study protocol driven, robust. Case Report Form Clinical Trial.
From www.xfanzexpo.com
Case Eport Form Electronic Qolty Format Ppt In Clinical With Trial Case Report Form Clinical Trial It's a structured document used. a case report form (crf) is a critical component of clinical trials and research studies. what is a case report form? A crf is a set of documents that collects data and information from a clinical trial. the case report form (crf) is a pivotal tool in clinical research. case report. Case Report Form Clinical Trial.
From www.scientific.net
Based Case Report Form Design for Clinical Trial Case Report Form Clinical Trial It's a structured document used. A crf is a set of documents that collects data and information from a clinical trial. what is a case report form? It is a document used in clinical trials to collect data from each. case report form (crf) is a specialized document in clinical research. the case report form (crf) is. Case Report Form Clinical Trial.
From www.xfanzexpo.com
Form E Report E2 80 93 Riat Support Center Crf Templates Intended For Case Report Form Clinical Trial a case report form (crf) is designed to collect the patient data in a clinical trial; what is a case report form? It is a document used in clinical trials to collect data from each. case report form (crf) is a specialized document in clinical research. a case report form (crf) is a critical component of. Case Report Form Clinical Trial.
From www.vrogue.co
Case Report Form Template Clinical Trials Large Study vrogue.co Case Report Form Clinical Trial a case report form (crf) is designed to collect the patient data in a clinical trial; It should be study protocol driven, robust in content. The crf is used by the study sponsor to capture. A crf is a set of documents that collects data and information from a clinical trial. the case report form (crf) is a. Case Report Form Clinical Trial.
From newcreativetemplateideas.blogspot.com
Case Report Form Template New Creative Template Ideas Case Report Form Clinical Trial case report form (crf) is a specialized document in clinical research. a case report form (crf) is a critical component of clinical trials and research studies. The crf is used by the study sponsor to capture. A crf is a set of documents that collects data and information from a clinical trial. It's a structured document used. . Case Report Form Clinical Trial.
From www.sampleforms.com
FREE 15+ Case Report Forms in PDF MS Word Case Report Form Clinical Trial A crf is a set of documents that collects data and information from a clinical trial. The crf is used by the study sponsor to capture. It is a document used in clinical trials to collect data from each. a case report form (crf) is a critical component of clinical trials and research studies. what is a case. Case Report Form Clinical Trial.
From besttemplate-ideas.blogspot.com
Case Report Form Template Clinical Trials Best Template Ideas Case Report Form Clinical Trial a case report form (crf) is designed to collect the patient data in a clinical trial; case report form (crf) is a specialized document in clinical research. It's a structured document used. 1.2 the case report form (crf) is a data collection tool used to capture the required data, as defined by the protocol, for each. It. Case Report Form Clinical Trial.
From www.smartsheet.com
Free Clinical Trial Templates Smartsheet Case Report Form Clinical Trial It should be study protocol driven, robust in content. It's a structured document used. what is a case report form? 1.2 the case report form (crf) is a data collection tool used to capture the required data, as defined by the protocol, for each. the case report form (crf) is a pivotal tool in clinical research. . Case Report Form Clinical Trial.
From www.researchgate.net
Clinical trial case identification and classification results. ABC Case Report Form Clinical Trial A crf is a set of documents that collects data and information from a clinical trial. the case report form (crf) is a pivotal tool in clinical research. case report form (crf) is a specialized document in clinical research. a case report form (crf) is a critical component of clinical trials and research studies. The crf is. Case Report Form Clinical Trial.
From documents.thegreenerleithsocial.org
Case Report Form Template Clinical Trials Documents Case Report Form Clinical Trial It's a structured document used. 1.2 the case report form (crf) is a data collection tool used to capture the required data, as defined by the protocol, for each. what is a case report form? a case report form (crf) is designed to collect the patient data in a clinical trial; A crf is a set of. Case Report Form Clinical Trial.