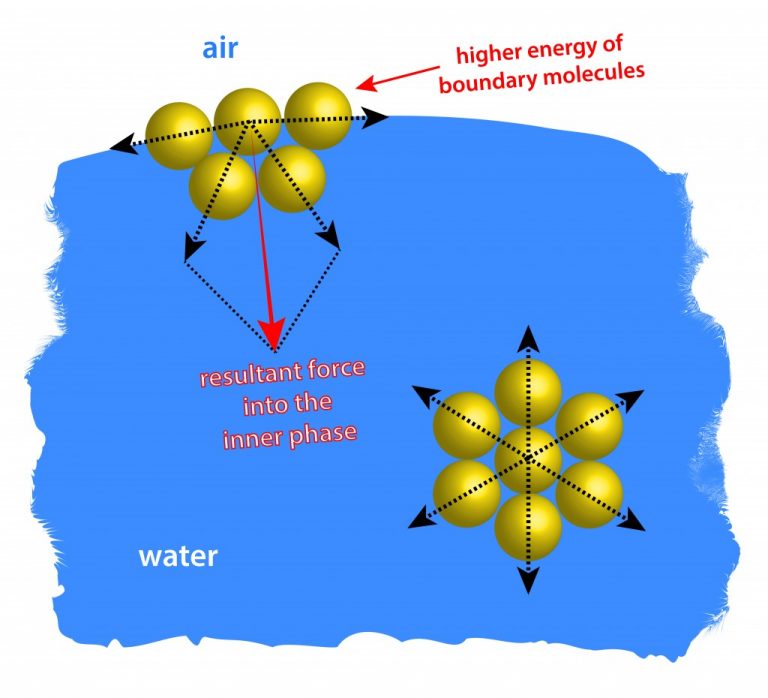
How Does Surface Tension Work In Nature . The weight of the needle is pulling the surface downward; Surface tension is responsible for the shape of. Suppose the cohesive forces are less than the adhesive forces. An example is mercury, whose surface tension at 25 °c is 485 mn/m. At the same time, the surface tension is pulling it up, suspending it on the surface of. The tendency to minimize that wall tension pulls the bubbles into spherical shapes. In that case, the liquid will spread out, thus,. Surface tension, property of a liquid surface displayed by its acting as if it were a stretched elastic membrane. Surface tension is a manifestation of the presence of the hydrogen bond. The value of surface tension for a particular liquid can be affected by the nature of the liquid, its surroundings, and the purity of the liquid. Those molecules of water that are at the surface are strongly attracted to. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces.
from www.scienceabc.com
Suppose the cohesive forces are less than the adhesive forces. Surface tension, property of a liquid surface displayed by its acting as if it were a stretched elastic membrane. The weight of the needle is pulling the surface downward; Those molecules of water that are at the surface are strongly attracted to. An example is mercury, whose surface tension at 25 °c is 485 mn/m. Surface tension is responsible for the shape of. The value of surface tension for a particular liquid can be affected by the nature of the liquid, its surroundings, and the purity of the liquid. The tendency to minimize that wall tension pulls the bubbles into spherical shapes. Surface tension is a manifestation of the presence of the hydrogen bond. At the same time, the surface tension is pulling it up, suspending it on the surface of.
Surface Tension Definition, Explanation, Examples And Significance
How Does Surface Tension Work In Nature Surface tension is responsible for the shape of. The weight of the needle is pulling the surface downward; Those molecules of water that are at the surface are strongly attracted to. Surface tension is a manifestation of the presence of the hydrogen bond. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. Surface tension is responsible for the shape of. In that case, the liquid will spread out, thus,. At the same time, the surface tension is pulling it up, suspending it on the surface of. The value of surface tension for a particular liquid can be affected by the nature of the liquid, its surroundings, and the purity of the liquid. Suppose the cohesive forces are less than the adhesive forces. Surface tension, property of a liquid surface displayed by its acting as if it were a stretched elastic membrane. An example is mercury, whose surface tension at 25 °c is 485 mn/m. The tendency to minimize that wall tension pulls the bubbles into spherical shapes.
From www.youtube.com
Surface Tension YouTube How Does Surface Tension Work In Nature In that case, the liquid will spread out, thus,. Surface tension, property of a liquid surface displayed by its acting as if it were a stretched elastic membrane. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. The value of surface tension for a particular liquid can be affected. How Does Surface Tension Work In Nature.
From www.thoughtco.com
Surface Tension Definition in Chemistry How Does Surface Tension Work In Nature The weight of the needle is pulling the surface downward; Surface tension is a manifestation of the presence of the hydrogen bond. Surface tension is responsible for the shape of. Surface tension, property of a liquid surface displayed by its acting as if it were a stretched elastic membrane. At the same time, the surface tension is pulling it up,. How Does Surface Tension Work In Nature.
From proper-cooking.info
Surface Tension Examples In Nature How Does Surface Tension Work In Nature Surface tension is a manifestation of the presence of the hydrogen bond. In that case, the liquid will spread out, thus,. The value of surface tension for a particular liquid can be affected by the nature of the liquid, its surroundings, and the purity of the liquid. Suppose the cohesive forces are less than the adhesive forces. An example is. How Does Surface Tension Work In Nature.
From funsizephysics.com
What is Surface Tension? FunsizePhysics How Does Surface Tension Work In Nature In that case, the liquid will spread out, thus,. Surface tension, property of a liquid surface displayed by its acting as if it were a stretched elastic membrane. The tendency to minimize that wall tension pulls the bubbles into spherical shapes. Surface tension is responsible for the shape of. The value of surface tension for a particular liquid can be. How Does Surface Tension Work In Nature.
From www.youtube.com
How does Surface Tension work? YouTube How Does Surface Tension Work In Nature The value of surface tension for a particular liquid can be affected by the nature of the liquid, its surroundings, and the purity of the liquid. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. In that case, the liquid will spread out, thus,. An example is mercury, whose. How Does Surface Tension Work In Nature.
From brainly.in
Please help. No spam. I will report. Thank you. Use photo down below to How Does Surface Tension Work In Nature The tendency to minimize that wall tension pulls the bubbles into spherical shapes. An example is mercury, whose surface tension at 25 °c is 485 mn/m. At the same time, the surface tension is pulling it up, suspending it on the surface of. Those molecules of water that are at the surface are strongly attracted to. Surface tension, property of. How Does Surface Tension Work In Nature.
From www.sciencefacts.net
Surface Tension Definition, Examples, and Unit How Does Surface Tension Work In Nature Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. Suppose the cohesive forces are less than the adhesive forces. Surface tension is a manifestation of the presence of the hydrogen bond. At the same time, the surface tension is pulling it up, suspending it on the surface of. An. How Does Surface Tension Work In Nature.
From byjus.com
Explain the surface tension phenomenon with examples. How Does Surface Tension Work In Nature Surface tension is responsible for the shape of. The value of surface tension for a particular liquid can be affected by the nature of the liquid, its surroundings, and the purity of the liquid. Surface tension, property of a liquid surface displayed by its acting as if it were a stretched elastic membrane. Suppose the cohesive forces are less than. How Does Surface Tension Work In Nature.
From www.scienceabc.com
Surface Tension Definition, Explanation, Examples And Significance How Does Surface Tension Work In Nature Those molecules of water that are at the surface are strongly attracted to. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. In that case, the liquid will spread out, thus,. The weight of the needle is pulling the surface downward; Surface tension is responsible for the shape of.. How Does Surface Tension Work In Nature.
From www.all-science-fair-projects.com
Investigating Water Surface Tension Complete Science Fair Projects How Does Surface Tension Work In Nature The value of surface tension for a particular liquid can be affected by the nature of the liquid, its surroundings, and the purity of the liquid. Suppose the cohesive forces are less than the adhesive forces. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. Surface tension, property of. How Does Surface Tension Work In Nature.
From www.australianenvironmentaleducation.com.au
Water is found everywhere on earth, so why is it important? How Does Surface Tension Work In Nature Those molecules of water that are at the surface are strongly attracted to. The tendency to minimize that wall tension pulls the bubbles into spherical shapes. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. Surface tension is responsible for the shape of. In that case, the liquid will. How Does Surface Tension Work In Nature.
From mavink.com
Surface Tension Animation How Does Surface Tension Work In Nature An example is mercury, whose surface tension at 25 °c is 485 mn/m. In that case, the liquid will spread out, thus,. Those molecules of water that are at the surface are strongly attracted to. The weight of the needle is pulling the surface downward; The value of surface tension for a particular liquid can be affected by the nature. How Does Surface Tension Work In Nature.
From gioenbudo.blob.core.windows.net
How Does Surface Tension Affect The Functioning Of Living Organisms at How Does Surface Tension Work In Nature Those molecules of water that are at the surface are strongly attracted to. The weight of the needle is pulling the surface downward; Surface tension is responsible for the shape of. The value of surface tension for a particular liquid can be affected by the nature of the liquid, its surroundings, and the purity of the liquid. The tendency to. How Does Surface Tension Work In Nature.
From mungfali.com
Water Surface Tension Bubble How Does Surface Tension Work In Nature Surface tension is a manifestation of the presence of the hydrogen bond. Surface tension is responsible for the shape of. The weight of the needle is pulling the surface downward; The value of surface tension for a particular liquid can be affected by the nature of the liquid, its surroundings, and the purity of the liquid. Those molecules of water. How Does Surface Tension Work In Nature.
From www.photocase.com
surface tension Nature a Royalty Free Stock Photo from Photocase How Does Surface Tension Work In Nature At the same time, the surface tension is pulling it up, suspending it on the surface of. Suppose the cohesive forces are less than the adhesive forces. The weight of the needle is pulling the surface downward; Surface tension is a manifestation of the presence of the hydrogen bond. Those molecules of water that are at the surface are strongly. How Does Surface Tension Work In Nature.
From cynthianalibrary.org
Surface Tension Sink or Float? How Does Surface Tension Work In Nature Surface tension is a manifestation of the presence of the hydrogen bond. An example is mercury, whose surface tension at 25 °c is 485 mn/m. The weight of the needle is pulling the surface downward; Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. Surface tension, property of a. How Does Surface Tension Work In Nature.
From www.youtube.com
Surface Tension What is it, how does it form, what properties does it How Does Surface Tension Work In Nature The value of surface tension for a particular liquid can be affected by the nature of the liquid, its surroundings, and the purity of the liquid. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. Surface tension is responsible for the shape of. Surface tension, property of a liquid. How Does Surface Tension Work In Nature.
From chem.libretexts.org
Surface Tension Chemistry LibreTexts How Does Surface Tension Work In Nature The weight of the needle is pulling the surface downward; Surface tension, property of a liquid surface displayed by its acting as if it were a stretched elastic membrane. At the same time, the surface tension is pulling it up, suspending it on the surface of. In that case, the liquid will spread out, thus,. Surface tension is responsible for. How Does Surface Tension Work In Nature.
From worksheethaunches.z14.web.core.windows.net
Surface Tension Explained For Kids How Does Surface Tension Work In Nature Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. Suppose the cohesive forces are less than the adhesive forces. Those molecules of water that are at the surface are strongly attracted to. Surface tension is a manifestation of the presence of the hydrogen bond. Surface tension is responsible for. How Does Surface Tension Work In Nature.
From www.youtube.com
Why is surface tension important? YouTube How Does Surface Tension Work In Nature The value of surface tension for a particular liquid can be affected by the nature of the liquid, its surroundings, and the purity of the liquid. The weight of the needle is pulling the surface downward; In that case, the liquid will spread out, thus,. Surface tension is responsible for the shape of. The tendency to minimize that wall tension. How Does Surface Tension Work In Nature.
From www.youtube.com
Surface Tension, part 2 Lecture 1.4 Chemical Engineering Fluid How Does Surface Tension Work In Nature The weight of the needle is pulling the surface downward; In that case, the liquid will spread out, thus,. At the same time, the surface tension is pulling it up, suspending it on the surface of. The tendency to minimize that wall tension pulls the bubbles into spherical shapes. Surface tension is a manifestation of the presence of the hydrogen. How Does Surface Tension Work In Nature.
From www.youtube.com
Surface Tension of Water Explained YouTube How Does Surface Tension Work In Nature The tendency to minimize that wall tension pulls the bubbles into spherical shapes. Suppose the cohesive forces are less than the adhesive forces. The weight of the needle is pulling the surface downward; Surface tension is responsible for the shape of. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular. How Does Surface Tension Work In Nature.
From www.skytechosting.com
What is Surface Tension? (With 5 examples) SkyTechosting How Does Surface Tension Work In Nature In that case, the liquid will spread out, thus,. The tendency to minimize that wall tension pulls the bubbles into spherical shapes. Surface tension is responsible for the shape of. The value of surface tension for a particular liquid can be affected by the nature of the liquid, its surroundings, and the purity of the liquid. The weight of the. How Does Surface Tension Work In Nature.
From www.geeksforgeeks.org
Surface Tension Definition, Formula, Causes, Examples, and FAQs How Does Surface Tension Work In Nature Surface tension is responsible for the shape of. In that case, the liquid will spread out, thus,. Surface tension, property of a liquid surface displayed by its acting as if it were a stretched elastic membrane. An example is mercury, whose surface tension at 25 °c is 485 mn/m. Surface tension is the energy, or work, required to increase the. How Does Surface Tension Work In Nature.
From www.purechemistry.org
SURFACE TENSION Purechemistry How Does Surface Tension Work In Nature Suppose the cohesive forces are less than the adhesive forces. Surface tension is a manifestation of the presence of the hydrogen bond. The tendency to minimize that wall tension pulls the bubbles into spherical shapes. Surface tension is responsible for the shape of. Surface tension, property of a liquid surface displayed by its acting as if it were a stretched. How Does Surface Tension Work In Nature.
From stock.adobe.com
illustration of physics, Surface tension of water, the cohesive forces How Does Surface Tension Work In Nature Suppose the cohesive forces are less than the adhesive forces. The tendency to minimize that wall tension pulls the bubbles into spherical shapes. The weight of the needle is pulling the surface downward; Surface tension, property of a liquid surface displayed by its acting as if it were a stretched elastic membrane. Surface tension is the energy, or work, required. How Does Surface Tension Work In Nature.
From www.youtube.com
surface tensionintermolecular forcessurface energyproperties of How Does Surface Tension Work In Nature At the same time, the surface tension is pulling it up, suspending it on the surface of. The weight of the needle is pulling the surface downward; Surface tension, property of a liquid surface displayed by its acting as if it were a stretched elastic membrane. Surface tension is the energy, or work, required to increase the surface area of. How Does Surface Tension Work In Nature.
From ar.inspiredpencil.com
Transpiration Stream Diagram How Does Surface Tension Work In Nature Suppose the cohesive forces are less than the adhesive forces. Surface tension, property of a liquid surface displayed by its acting as if it were a stretched elastic membrane. In that case, the liquid will spread out, thus,. Surface tension is a manifestation of the presence of the hydrogen bond. An example is mercury, whose surface tension at 25 °c. How Does Surface Tension Work In Nature.
From www.elephango.com
Tension in the Water Educational Resources K12 Learning, Physical How Does Surface Tension Work In Nature An example is mercury, whose surface tension at 25 °c is 485 mn/m. Those molecules of water that are at the surface are strongly attracted to. Surface tension, property of a liquid surface displayed by its acting as if it were a stretched elastic membrane. Surface tension is responsible for the shape of. At the same time, the surface tension. How Does Surface Tension Work In Nature.
From www.biolinscientific.com
Surface tension of water Why is it so high? How Does Surface Tension Work In Nature Surface tension is responsible for the shape of. Surface tension, property of a liquid surface displayed by its acting as if it were a stretched elastic membrane. The weight of the needle is pulling the surface downward; Surface tension is a manifestation of the presence of the hydrogen bond. An example is mercury, whose surface tension at 25 °c is. How Does Surface Tension Work In Nature.
From www.thoughtco.com
What Is Surface Tension? Definition and Experiments How Does Surface Tension Work In Nature The value of surface tension for a particular liquid can be affected by the nature of the liquid, its surroundings, and the purity of the liquid. An example is mercury, whose surface tension at 25 °c is 485 mn/m. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. Surface. How Does Surface Tension Work In Nature.
From exoxqkwlq.blob.core.windows.net
How Does Surface Tension Relate To Water at Ron Lowery blog How Does Surface Tension Work In Nature The tendency to minimize that wall tension pulls the bubbles into spherical shapes. In that case, the liquid will spread out, thus,. Surface tension is responsible for the shape of. Suppose the cohesive forces are less than the adhesive forces. Surface tension is a manifestation of the presence of the hydrogen bond. The value of surface tension for a particular. How Does Surface Tension Work In Nature.
From www.slideserve.com
PPT Chapter 2 Properties of Fluids PowerPoint Presentation, free How Does Surface Tension Work In Nature Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. In that case, the liquid will spread out, thus,. The tendency to minimize that wall tension pulls the bubbles into spherical shapes. An example is mercury, whose surface tension at 25 °c is 485 mn/m. Surface tension is responsible for. How Does Surface Tension Work In Nature.
From fyozfaljz.blob.core.windows.net
How Does Surface Tension Affect Humans at Andrew Rex blog How Does Surface Tension Work In Nature Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. In that case, the liquid will spread out, thus,. Suppose the cohesive forces are less than the adhesive forces. Surface tension is responsible for the shape of. The weight of the needle is pulling the surface downward; The value of. How Does Surface Tension Work In Nature.
From psiberg.com
Surface Energy vs. Tension The Key Differences and Examples How Does Surface Tension Work In Nature Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. Surface tension is responsible for the shape of. Those molecules of water that are at the surface are strongly attracted to. Surface tension is a manifestation of the presence of the hydrogen bond. The value of surface tension for a. How Does Surface Tension Work In Nature.