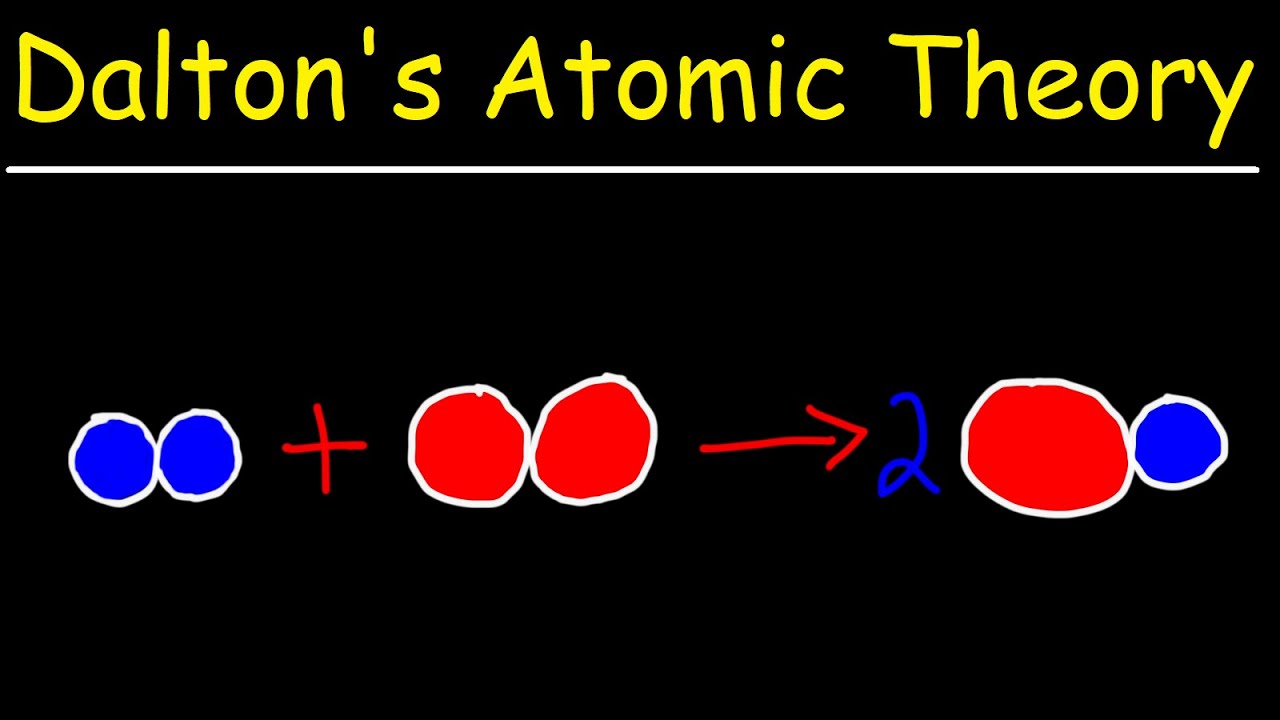
Dalton Mass Of An Atom . Atoms consist of a nucleus containing protons and neutrons, surrounded by electrons in shells. The numbers of particles in an atom can be calculated. Dalton states that atoms of a given element have precisely the same masses. Since 1961 the standard unit of. The concepts of this foundation include the atomic theory, the composition and mass of an atom, the variability of the composition of isotopes, ion. However, it was later established that atoms of the same element can have different masses. 121 rows atomic weight, ratio of the average mass of a chemical element’s atoms to some standard. For example, hydrogen, deuterium, and tritium are isotopes with different masses. They are known as isotopes. The atomic mass is usually measured in the units unified atomic mass unit (u), or dalton (da). 1 u = 1 da = m(12 c)/12
from www.youtube.com
The atomic mass is usually measured in the units unified atomic mass unit (u), or dalton (da). For example, hydrogen, deuterium, and tritium are isotopes with different masses. Atoms consist of a nucleus containing protons and neutrons, surrounded by electrons in shells. 1 u = 1 da = m(12 c)/12 The numbers of particles in an atom can be calculated. Dalton states that atoms of a given element have precisely the same masses. However, it was later established that atoms of the same element can have different masses. Since 1961 the standard unit of. 121 rows atomic weight, ratio of the average mass of a chemical element’s atoms to some standard. The concepts of this foundation include the atomic theory, the composition and mass of an atom, the variability of the composition of isotopes, ion.
Dalton's Atomic Theory YouTube
Dalton Mass Of An Atom The atomic mass is usually measured in the units unified atomic mass unit (u), or dalton (da). The atomic mass is usually measured in the units unified atomic mass unit (u), or dalton (da). 1 u = 1 da = m(12 c)/12 Atoms consist of a nucleus containing protons and neutrons, surrounded by electrons in shells. The numbers of particles in an atom can be calculated. Dalton states that atoms of a given element have precisely the same masses. Since 1961 the standard unit of. However, it was later established that atoms of the same element can have different masses. They are known as isotopes. For example, hydrogen, deuterium, and tritium are isotopes with different masses. The concepts of this foundation include the atomic theory, the composition and mass of an atom, the variability of the composition of isotopes, ion. 121 rows atomic weight, ratio of the average mass of a chemical element’s atoms to some standard.
From www.slideserve.com
PPT Chapter 4 Chemical Foundations Elements, Atoms, and Ions Dalton Mass Of An Atom The atomic mass is usually measured in the units unified atomic mass unit (u), or dalton (da). The numbers of particles in an atom can be calculated. For example, hydrogen, deuterium, and tritium are isotopes with different masses. The concepts of this foundation include the atomic theory, the composition and mass of an atom, the variability of the composition of. Dalton Mass Of An Atom.
From www.teachoo.com
Dalton's Atomic Theory Postulate, Limitations [Teachoo] Dalton Mass Of An Atom The atomic mass is usually measured in the units unified atomic mass unit (u), or dalton (da). The concepts of this foundation include the atomic theory, the composition and mass of an atom, the variability of the composition of isotopes, ion. They are known as isotopes. The numbers of particles in an atom can be calculated. Dalton states that atoms. Dalton Mass Of An Atom.
From www.teachoo.com
Dalton's Atomic Theory Postulate, Limitations [Teachoo] Dalton Mass Of An Atom For example, hydrogen, deuterium, and tritium are isotopes with different masses. 121 rows atomic weight, ratio of the average mass of a chemical element’s atoms to some standard. Dalton states that atoms of a given element have precisely the same masses. However, it was later established that atoms of the same element can have different masses. Since 1961 the standard. Dalton Mass Of An Atom.
From www.slideserve.com
PPT John Dalton PowerPoint Presentation ID3878263 Dalton Mass Of An Atom 121 rows atomic weight, ratio of the average mass of a chemical element’s atoms to some standard. 1 u = 1 da = m(12 c)/12 The concepts of this foundation include the atomic theory, the composition and mass of an atom, the variability of the composition of isotopes, ion. They are known as isotopes. For example, hydrogen, deuterium, and tritium. Dalton Mass Of An Atom.
From www.slideserve.com
PPT Chapter 4 Atoms PowerPoint Presentation, free download ID5528711 Dalton Mass Of An Atom For example, hydrogen, deuterium, and tritium are isotopes with different masses. The concepts of this foundation include the atomic theory, the composition and mass of an atom, the variability of the composition of isotopes, ion. The numbers of particles in an atom can be calculated. They are known as isotopes. Atoms consist of a nucleus containing protons and neutrons, surrounded. Dalton Mass Of An Atom.
From www.youtube.com
Ch 1.5 Law of Multiple proportions, Daltons Atomic Theory YouTube Dalton Mass Of An Atom However, it was later established that atoms of the same element can have different masses. They are known as isotopes. 1 u = 1 da = m(12 c)/12 The concepts of this foundation include the atomic theory, the composition and mass of an atom, the variability of the composition of isotopes, ion. The atomic mass is usually measured in the. Dalton Mass Of An Atom.
From time.graphics
John Dalton's Atomic Model (jan 1, 1803 dec 31, 1803) (Timeline) Dalton Mass Of An Atom For example, hydrogen, deuterium, and tritium are isotopes with different masses. The numbers of particles in an atom can be calculated. 121 rows atomic weight, ratio of the average mass of a chemical element’s atoms to some standard. Since 1961 the standard unit of. However, it was later established that atoms of the same element can have different masses. They. Dalton Mass Of An Atom.
From www.scienceworlds.xyz
Atomic theories Dalton Mass Of An Atom The concepts of this foundation include the atomic theory, the composition and mass of an atom, the variability of the composition of isotopes, ion. 1 u = 1 da = m(12 c)/12 They are known as isotopes. The numbers of particles in an atom can be calculated. The atomic mass is usually measured in the units unified atomic mass unit. Dalton Mass Of An Atom.
From www.youtube.com
Dalton's Atomic Theory YouTube Dalton Mass Of An Atom The concepts of this foundation include the atomic theory, the composition and mass of an atom, the variability of the composition of isotopes, ion. 1 u = 1 da = m(12 c)/12 Dalton states that atoms of a given element have precisely the same masses. The atomic mass is usually measured in the units unified atomic mass unit (u), or. Dalton Mass Of An Atom.
From atomictheorysquad.weebly.com
Dalton Atomic Theory Dalton Mass Of An Atom 1 u = 1 da = m(12 c)/12 Since 1961 the standard unit of. Atoms consist of a nucleus containing protons and neutrons, surrounded by electrons in shells. They are known as isotopes. However, it was later established that atoms of the same element can have different masses. The numbers of particles in an atom can be calculated. 121 rows. Dalton Mass Of An Atom.
From www.sliderbase.com
The ATOM Presentation Chemistry Dalton Mass Of An Atom The atomic mass is usually measured in the units unified atomic mass unit (u), or dalton (da). For example, hydrogen, deuterium, and tritium are isotopes with different masses. The concepts of this foundation include the atomic theory, the composition and mass of an atom, the variability of the composition of isotopes, ion. Dalton states that atoms of a given element. Dalton Mass Of An Atom.
From www.britannica.com
John Dalton's atomic theory explained Britannica Dalton Mass Of An Atom They are known as isotopes. Atoms consist of a nucleus containing protons and neutrons, surrounded by electrons in shells. However, it was later established that atoms of the same element can have different masses. For example, hydrogen, deuterium, and tritium are isotopes with different masses. The numbers of particles in an atom can be calculated. The atomic mass is usually. Dalton Mass Of An Atom.
From www.slideserve.com
PPT Chapter 4 Atomic Structure PowerPoint Presentation, free download Dalton Mass Of An Atom Atoms consist of a nucleus containing protons and neutrons, surrounded by electrons in shells. The numbers of particles in an atom can be calculated. Since 1961 the standard unit of. Dalton states that atoms of a given element have precisely the same masses. 121 rows atomic weight, ratio of the average mass of a chemical element’s atoms to some standard.. Dalton Mass Of An Atom.
From www.animalia-life.club
Daltons Atomic Model Labeled Dalton Mass Of An Atom Since 1961 the standard unit of. 121 rows atomic weight, ratio of the average mass of a chemical element’s atoms to some standard. Atoms consist of a nucleus containing protons and neutrons, surrounded by electrons in shells. 1 u = 1 da = m(12 c)/12 However, it was later established that atoms of the same element can have different masses.. Dalton Mass Of An Atom.
From www.animalia-life.club
Daltons Atomic Model Labeled Dalton Mass Of An Atom Dalton states that atoms of a given element have precisely the same masses. 1 u = 1 da = m(12 c)/12 The numbers of particles in an atom can be calculated. However, it was later established that atoms of the same element can have different masses. The concepts of this foundation include the atomic theory, the composition and mass of. Dalton Mass Of An Atom.
From www.slideserve.com
PPT Atomic Structure PowerPoint Presentation, free download ID9720990 Dalton Mass Of An Atom 121 rows atomic weight, ratio of the average mass of a chemical element’s atoms to some standard. Atoms consist of a nucleus containing protons and neutrons, surrounded by electrons in shells. The atomic mass is usually measured in the units unified atomic mass unit (u), or dalton (da). For example, hydrogen, deuterium, and tritium are isotopes with different masses. 1. Dalton Mass Of An Atom.
From www.slideserve.com
PPT Atoms, Molecules, and Ions PowerPoint Presentation, free download Dalton Mass Of An Atom Since 1961 the standard unit of. Dalton states that atoms of a given element have precisely the same masses. 1 u = 1 da = m(12 c)/12 They are known as isotopes. However, it was later established that atoms of the same element can have different masses. The atomic mass is usually measured in the units unified atomic mass unit. Dalton Mass Of An Atom.
From slideplayer.com
Chapter 2 The Chemical Level of Organization ppt download Dalton Mass Of An Atom 1 u = 1 da = m(12 c)/12 The atomic mass is usually measured in the units unified atomic mass unit (u), or dalton (da). They are known as isotopes. Atoms consist of a nucleus containing protons and neutrons, surrounded by electrons in shells. The numbers of particles in an atom can be calculated. Since 1961 the standard unit of.. Dalton Mass Of An Atom.
From www.worksheetsplanet.com
Dalton's Atomic Theory Dalton Mass Of An Atom The numbers of particles in an atom can be calculated. 121 rows atomic weight, ratio of the average mass of a chemical element’s atoms to some standard. They are known as isotopes. The concepts of this foundation include the atomic theory, the composition and mass of an atom, the variability of the composition of isotopes, ion. Dalton states that atoms. Dalton Mass Of An Atom.
From www.jagranjosh.com
Dalton’s Atomic Theory Know in detail here Dalton Mass Of An Atom However, it was later established that atoms of the same element can have different masses. The numbers of particles in an atom can be calculated. They are known as isotopes. 1 u = 1 da = m(12 c)/12 For example, hydrogen, deuterium, and tritium are isotopes with different masses. The atomic mass is usually measured in the units unified atomic. Dalton Mass Of An Atom.
From www.youtube.com
CHEMISTRY 101 The three laws that led to Daltons Atomic Theory YouTube Dalton Mass Of An Atom However, it was later established that atoms of the same element can have different masses. Since 1961 the standard unit of. 1 u = 1 da = m(12 c)/12 The numbers of particles in an atom can be calculated. The atomic mass is usually measured in the units unified atomic mass unit (u), or dalton (da). The concepts of this. Dalton Mass Of An Atom.
From www.ck12.org
Dalton's Atomic Theory ( Read ) Physical Science CK12 Foundation Dalton Mass Of An Atom The concepts of this foundation include the atomic theory, the composition and mass of an atom, the variability of the composition of isotopes, ion. However, it was later established that atoms of the same element can have different masses. The numbers of particles in an atom can be calculated. 121 rows atomic weight, ratio of the average mass of a. Dalton Mass Of An Atom.
From www.sciencephoto.com
Dalton's list of atomic and molecular symbols Stock Image V600/0027 Dalton Mass Of An Atom The concepts of this foundation include the atomic theory, the composition and mass of an atom, the variability of the composition of isotopes, ion. They are known as isotopes. The numbers of particles in an atom can be calculated. Since 1961 the standard unit of. The atomic mass is usually measured in the units unified atomic mass unit (u), or. Dalton Mass Of An Atom.
From graphiceducation.com.au
Dalton's Atomic Theory Graphic Education Dalton Mass Of An Atom The atomic mass is usually measured in the units unified atomic mass unit (u), or dalton (da). They are known as isotopes. 121 rows atomic weight, ratio of the average mass of a chemical element’s atoms to some standard. Dalton states that atoms of a given element have precisely the same masses. For example, hydrogen, deuterium, and tritium are isotopes. Dalton Mass Of An Atom.
From www.teachoo.com
Dalton's Atomic Theory Postulate, Limitations [Teachoo] Dalton Mass Of An Atom However, it was later established that atoms of the same element can have different masses. The atomic mass is usually measured in the units unified atomic mass unit (u), or dalton (da). 121 rows atomic weight, ratio of the average mass of a chemical element’s atoms to some standard. They are known as isotopes. Dalton states that atoms of a. Dalton Mass Of An Atom.
From www.slideshare.net
Dalton’s Atomic Theory Dalton Mass Of An Atom 121 rows atomic weight, ratio of the average mass of a chemical element’s atoms to some standard. 1 u = 1 da = m(12 c)/12 However, it was later established that atoms of the same element can have different masses. For example, hydrogen, deuterium, and tritium are isotopes with different masses. They are known as isotopes. The concepts of this. Dalton Mass Of An Atom.
From www.youtube.com
Dalton's Atomic Theory YouTube Dalton Mass Of An Atom The numbers of particles in an atom can be calculated. The atomic mass is usually measured in the units unified atomic mass unit (u), or dalton (da). Atoms consist of a nucleus containing protons and neutrons, surrounded by electrons in shells. 121 rows atomic weight, ratio of the average mass of a chemical element’s atoms to some standard. However, it. Dalton Mass Of An Atom.
From www.slideserve.com
PPT Chapter 4 Atomic Structure PowerPoint Presentation, free download Dalton Mass Of An Atom However, it was later established that atoms of the same element can have different masses. The numbers of particles in an atom can be calculated. 1 u = 1 da = m(12 c)/12 For example, hydrogen, deuterium, and tritium are isotopes with different masses. Atoms consist of a nucleus containing protons and neutrons, surrounded by electrons in shells. The concepts. Dalton Mass Of An Atom.
From www.youtube.com
Dalton's Atomic Theory YouTube Dalton Mass Of An Atom However, it was later established that atoms of the same element can have different masses. The concepts of this foundation include the atomic theory, the composition and mass of an atom, the variability of the composition of isotopes, ion. 121 rows atomic weight, ratio of the average mass of a chemical element’s atoms to some standard. The numbers of particles. Dalton Mass Of An Atom.
From www.slideserve.com
PPT Atomic Structure PowerPoint Presentation, free download ID2711746 Dalton Mass Of An Atom Atoms consist of a nucleus containing protons and neutrons, surrounded by electrons in shells. They are known as isotopes. Since 1961 the standard unit of. The numbers of particles in an atom can be calculated. For example, hydrogen, deuterium, and tritium are isotopes with different masses. The atomic mass is usually measured in the units unified atomic mass unit (u),. Dalton Mass Of An Atom.
From www.politicalfunda.com
Atomic mass Atomic mass definition, Units, & Facts Dalton Mass Of An Atom Since 1961 the standard unit of. The numbers of particles in an atom can be calculated. However, it was later established that atoms of the same element can have different masses. 1 u = 1 da = m(12 c)/12 The atomic mass is usually measured in the units unified atomic mass unit (u), or dalton (da). They are known as. Dalton Mass Of An Atom.
From www.sliderbase.com
Atomic Structure Presentation Chemistry Dalton Mass Of An Atom For example, hydrogen, deuterium, and tritium are isotopes with different masses. They are known as isotopes. However, it was later established that atoms of the same element can have different masses. The atomic mass is usually measured in the units unified atomic mass unit (u), or dalton (da). 121 rows atomic weight, ratio of the average mass of a chemical. Dalton Mass Of An Atom.
From in.pinterest.com
Daltons Atomic Theory Easy Science Atomic theory, Chemical changes Dalton Mass Of An Atom 121 rows atomic weight, ratio of the average mass of a chemical element’s atoms to some standard. Since 1961 the standard unit of. 1 u = 1 da = m(12 c)/12 The concepts of this foundation include the atomic theory, the composition and mass of an atom, the variability of the composition of isotopes, ion. They are known as isotopes.. Dalton Mass Of An Atom.
From www.slideserve.com
PPT Chapter 2 Atoms, Molecules, and Ions PowerPoint Presentation Dalton Mass Of An Atom 1 u = 1 da = m(12 c)/12 However, it was later established that atoms of the same element can have different masses. Since 1961 the standard unit of. The concepts of this foundation include the atomic theory, the composition and mass of an atom, the variability of the composition of isotopes, ion. Atoms consist of a nucleus containing protons. Dalton Mass Of An Atom.
From ppt-online.org
Atomic theory and structure of an atom презентация онлайн Dalton Mass Of An Atom 121 rows atomic weight, ratio of the average mass of a chemical element’s atoms to some standard. For example, hydrogen, deuterium, and tritium are isotopes with different masses. The atomic mass is usually measured in the units unified atomic mass unit (u), or dalton (da). 1 u = 1 da = m(12 c)/12 They are known as isotopes. The concepts. Dalton Mass Of An Atom.