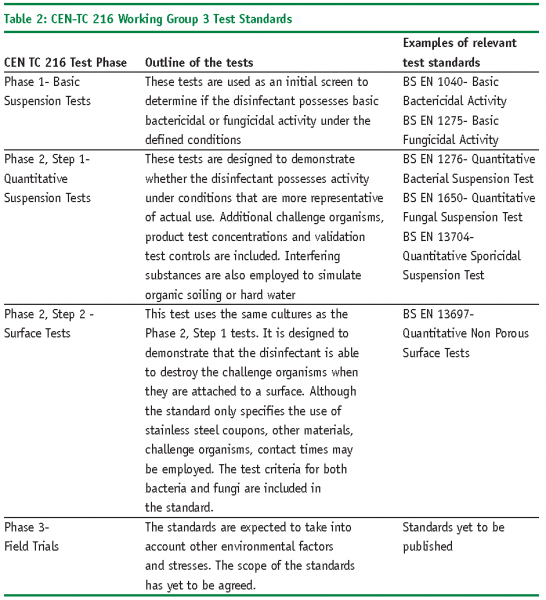
Disinfectant Validation Usp 1072 . The removal of residual disinfectants. Usp chapter disinfectants and antiseptics. Suspension testing evaluates efficacy of disinfectants under defined laboratory conditions. Disinfectants applied on potential product contact surfaces are typically removed with 70% alcohol wipes. The united states pharmacopoeia (usp) chapter 1072> [7] provides further advice on efficacy testing for the pharmaceutical sector, stating “to demonstrate the efficacy. The requirements for aseptic processing include readily cleanable floors, walls, and ceilings that have smooth and nonporous surfaces;. These are the appropriate acceptance criteria for disinfectant efficacy. Disinfectant—a chemical or physical agent that destroys or removes vegetative forms of harmful microorganisms when applied to a surface. Usp disinfectants and antiseptics calls for: Organisms dried onto a stainless steel surface.
from www.europeanpharmaceuticalreview.com
The requirements for aseptic processing include readily cleanable floors, walls, and ceilings that have smooth and nonporous surfaces;. Disinfectants applied on potential product contact surfaces are typically removed with 70% alcohol wipes. Disinfectant—a chemical or physical agent that destroys or removes vegetative forms of harmful microorganisms when applied to a surface. Usp disinfectants and antiseptics calls for: Usp chapter disinfectants and antiseptics. Organisms dried onto a stainless steel surface. These are the appropriate acceptance criteria for disinfectant efficacy. The united states pharmacopoeia (usp) chapter 1072> [7] provides further advice on efficacy testing for the pharmaceutical sector, stating “to demonstrate the efficacy. Suspension testing evaluates efficacy of disinfectants under defined laboratory conditions. The removal of residual disinfectants.
Disinfectant validation European Pharmaceutical Review
Disinfectant Validation Usp 1072 The removal of residual disinfectants. Disinfectant—a chemical or physical agent that destroys or removes vegetative forms of harmful microorganisms when applied to a surface. Organisms dried onto a stainless steel surface. Usp chapter disinfectants and antiseptics. These are the appropriate acceptance criteria for disinfectant efficacy. Usp disinfectants and antiseptics calls for: The requirements for aseptic processing include readily cleanable floors, walls, and ceilings that have smooth and nonporous surfaces;. The united states pharmacopoeia (usp) chapter 1072> [7] provides further advice on efficacy testing for the pharmaceutical sector, stating “to demonstrate the efficacy. The removal of residual disinfectants. Suspension testing evaluates efficacy of disinfectants under defined laboratory conditions. Disinfectants applied on potential product contact surfaces are typically removed with 70% alcohol wipes.
From www.scribd.com
Disinfectant Validation PDF Disinfectant Sterilization (Microbiology) Disinfectant Validation Usp 1072 Disinfectants applied on potential product contact surfaces are typically removed with 70% alcohol wipes. These are the appropriate acceptance criteria for disinfectant efficacy. Usp disinfectants and antiseptics calls for: Suspension testing evaluates efficacy of disinfectants under defined laboratory conditions. Organisms dried onto a stainless steel surface. The requirements for aseptic processing include readily cleanable floors, walls, and ceilings that have. Disinfectant Validation Usp 1072.
From www.scribd.com
Protocol For Disinfectant Validation Disinfectant Validation Protocol Disinfectant Validation Usp 1072 These are the appropriate acceptance criteria for disinfectant efficacy. Suspension testing evaluates efficacy of disinfectants under defined laboratory conditions. Usp chapter disinfectants and antiseptics. Usp disinfectants and antiseptics calls for: Disinfectant—a chemical or physical agent that destroys or removes vegetative forms of harmful microorganisms when applied to a surface. The united states pharmacopoeia (usp) chapter 1072> [7] provides further advice. Disinfectant Validation Usp 1072.
From www.scribd.com
Disinfectant Validation PDF Disinfectant Validation Usp 1072 The requirements for aseptic processing include readily cleanable floors, walls, and ceilings that have smooth and nonporous surfaces;. Disinfectant—a chemical or physical agent that destroys or removes vegetative forms of harmful microorganisms when applied to a surface. The united states pharmacopoeia (usp) chapter 1072> [7] provides further advice on efficacy testing for the pharmaceutical sector, stating “to demonstrate the efficacy.. Disinfectant Validation Usp 1072.
From www.europeanpharmaceuticalreview.com
Disinfectant validation European Pharmaceutical Review Disinfectant Validation Usp 1072 Usp disinfectants and antiseptics calls for: Suspension testing evaluates efficacy of disinfectants under defined laboratory conditions. The united states pharmacopoeia (usp) chapter 1072> [7] provides further advice on efficacy testing for the pharmaceutical sector, stating “to demonstrate the efficacy. Usp chapter disinfectants and antiseptics. Disinfectant—a chemical or physical agent that destroys or removes vegetative forms of harmful microorganisms when applied. Disinfectant Validation Usp 1072.
From www.scribd.com
SOP For Disinfectants Validation Report Form Form PDF Disinfectant Validation Usp 1072 Disinfectants applied on potential product contact surfaces are typically removed with 70% alcohol wipes. Usp disinfectants and antiseptics calls for: Organisms dried onto a stainless steel surface. The removal of residual disinfectants. The requirements for aseptic processing include readily cleanable floors, walls, and ceilings that have smooth and nonporous surfaces;. The united states pharmacopoeia (usp) chapter 1072> [7] provides further. Disinfectant Validation Usp 1072.
From www.pharmaguideline.com
Validation Protocol for Efficacy of Chemical Disinfectants Disinfectant Validation Usp 1072 The removal of residual disinfectants. Disinfectant—a chemical or physical agent that destroys or removes vegetative forms of harmful microorganisms when applied to a surface. The united states pharmacopoeia (usp) chapter 1072> [7] provides further advice on efficacy testing for the pharmaceutical sector, stating “to demonstrate the efficacy. The requirements for aseptic processing include readily cleanable floors, walls, and ceilings that. Disinfectant Validation Usp 1072.
From www.scribd.com
Principles of Disinfectant Validation PDF Disinfectant Disinfectant Validation Usp 1072 Disinfectant—a chemical or physical agent that destroys or removes vegetative forms of harmful microorganisms when applied to a surface. The united states pharmacopoeia (usp) chapter 1072> [7] provides further advice on efficacy testing for the pharmaceutical sector, stating “to demonstrate the efficacy. Usp disinfectants and antiseptics calls for: The removal of residual disinfectants. The requirements for aseptic processing include readily. Disinfectant Validation Usp 1072.
From thewitfire.in
Disinfectant Validation Protocol? Method of Disinfectant Validation? Disinfectant Validation Usp 1072 Disinfectants applied on potential product contact surfaces are typically removed with 70% alcohol wipes. The removal of residual disinfectants. These are the appropriate acceptance criteria for disinfectant efficacy. Disinfectant—a chemical or physical agent that destroys or removes vegetative forms of harmful microorganisms when applied to a surface. Usp disinfectants and antiseptics calls for: Organisms dried onto a stainless steel surface.. Disinfectant Validation Usp 1072.
From www.americanpharmaceuticalreview.com
InSitu Disinfectant Validation Case Study American Pharmaceutical Disinfectant Validation Usp 1072 Organisms dried onto a stainless steel surface. Usp disinfectants and antiseptics calls for: The requirements for aseptic processing include readily cleanable floors, walls, and ceilings that have smooth and nonporous surfaces;. Suspension testing evaluates efficacy of disinfectants under defined laboratory conditions. Disinfectant—a chemical or physical agent that destroys or removes vegetative forms of harmful microorganisms when applied to a surface.. Disinfectant Validation Usp 1072.
From www.laboratuar.net
Cleaning Validation and Disinfectant Efficiency Studies Disinfectant Validation Usp 1072 The removal of residual disinfectants. Usp disinfectants and antiseptics calls for: Suspension testing evaluates efficacy of disinfectants under defined laboratory conditions. These are the appropriate acceptance criteria for disinfectant efficacy. Disinfectants applied on potential product contact surfaces are typically removed with 70% alcohol wipes. Usp chapter disinfectants and antiseptics. The united states pharmacopoeia (usp) chapter 1072> [7] provides further advice. Disinfectant Validation Usp 1072.
From thewitfire.in
Disinfectant Rotation Validation Protocol? How does it Work? Disinfectant Validation Usp 1072 Organisms dried onto a stainless steel surface. The removal of residual disinfectants. The requirements for aseptic processing include readily cleanable floors, walls, and ceilings that have smooth and nonporous surfaces;. These are the appropriate acceptance criteria for disinfectant efficacy. Usp chapter disinfectants and antiseptics. Disinfectant—a chemical or physical agent that destroys or removes vegetative forms of harmful microorganisms when applied. Disinfectant Validation Usp 1072.
From www.europeanpharmaceuticalreview.com
Disinfectant validation European Pharmaceutical Review Disinfectant Validation Usp 1072 The removal of residual disinfectants. Disinfectants applied on potential product contact surfaces are typically removed with 70% alcohol wipes. The united states pharmacopoeia (usp) chapter 1072> [7] provides further advice on efficacy testing for the pharmaceutical sector, stating “to demonstrate the efficacy. Usp disinfectants and antiseptics calls for: The requirements for aseptic processing include readily cleanable floors, walls, and ceilings. Disinfectant Validation Usp 1072.
From es.scribd.com
USPNF 〈1072〉 Disinfectants and Antiseptics PDF Esterilización Disinfectant Validation Usp 1072 The removal of residual disinfectants. These are the appropriate acceptance criteria for disinfectant efficacy. Suspension testing evaluates efficacy of disinfectants under defined laboratory conditions. Disinfectants applied on potential product contact surfaces are typically removed with 70% alcohol wipes. The united states pharmacopoeia (usp) chapter 1072> [7] provides further advice on efficacy testing for the pharmaceutical sector, stating “to demonstrate the. Disinfectant Validation Usp 1072.
From www.scribd.com
Disinfectant Validation PDF Disinfectant Food And Drug Administration Disinfectant Validation Usp 1072 Usp disinfectants and antiseptics calls for: The removal of residual disinfectants. The united states pharmacopoeia (usp) chapter 1072> [7] provides further advice on efficacy testing for the pharmaceutical sector, stating “to demonstrate the efficacy. The requirements for aseptic processing include readily cleanable floors, walls, and ceilings that have smooth and nonporous surfaces;. These are the appropriate acceptance criteria for disinfectant. Disinfectant Validation Usp 1072.
From dokumen.tips
(PDF) Disinfectant Efficacy Testing and Validation Disinfectant Validation Usp 1072 Disinfectant—a chemical or physical agent that destroys or removes vegetative forms of harmful microorganisms when applied to a surface. The requirements for aseptic processing include readily cleanable floors, walls, and ceilings that have smooth and nonporous surfaces;. Organisms dried onto a stainless steel surface. The removal of residual disinfectants. Usp chapter disinfectants and antiseptics. Suspension testing evaluates efficacy of disinfectants. Disinfectant Validation Usp 1072.
From www.scribd.com
The ABCs of Disinfectant Validation PDF Disinfectant Antimicrobial Disinfectant Validation Usp 1072 The requirements for aseptic processing include readily cleanable floors, walls, and ceilings that have smooth and nonporous surfaces;. Organisms dried onto a stainless steel surface. Suspension testing evaluates efficacy of disinfectants under defined laboratory conditions. Usp chapter disinfectants and antiseptics. Usp disinfectants and antiseptics calls for: The united states pharmacopoeia (usp) chapter 1072> [7] provides further advice on efficacy testing. Disinfectant Validation Usp 1072.
From www.slideserve.com
PPT Summary of USP 797 for Compounding Sterile Preparations Disinfectant Validation Usp 1072 Disinfectants applied on potential product contact surfaces are typically removed with 70% alcohol wipes. The united states pharmacopoeia (usp) chapter 1072> [7] provides further advice on efficacy testing for the pharmaceutical sector, stating “to demonstrate the efficacy. Organisms dried onto a stainless steel surface. Disinfectant—a chemical or physical agent that destroys or removes vegetative forms of harmful microorganisms when applied. Disinfectant Validation Usp 1072.
From www.pharmasopworld.com
Disinfectant efficacy validation protocol Disinfectant Validation Usp 1072 The removal of residual disinfectants. Disinfectants applied on potential product contact surfaces are typically removed with 70% alcohol wipes. The requirements for aseptic processing include readily cleanable floors, walls, and ceilings that have smooth and nonporous surfaces;. Suspension testing evaluates efficacy of disinfectants under defined laboratory conditions. Organisms dried onto a stainless steel surface. These are the appropriate acceptance criteria. Disinfectant Validation Usp 1072.
From www.scribd.com
Validation Protocol For Efficacy of Chemical Disinfectants PDF Disinfectant Validation Usp 1072 The removal of residual disinfectants. The requirements for aseptic processing include readily cleanable floors, walls, and ceilings that have smooth and nonporous surfaces;. These are the appropriate acceptance criteria for disinfectant efficacy. Disinfectant—a chemical or physical agent that destroys or removes vegetative forms of harmful microorganisms when applied to a surface. Usp chapter disinfectants and antiseptics. Disinfectants applied on potential. Disinfectant Validation Usp 1072.
From projectclean.com
RTU Disinfectant Project Clean Disinfectant Validation Usp 1072 The united states pharmacopoeia (usp) chapter 1072> [7] provides further advice on efficacy testing for the pharmaceutical sector, stating “to demonstrate the efficacy. Disinfectants applied on potential product contact surfaces are typically removed with 70% alcohol wipes. The requirements for aseptic processing include readily cleanable floors, walls, and ceilings that have smooth and nonporous surfaces;. Suspension testing evaluates efficacy of. Disinfectant Validation Usp 1072.
From www.scribd.com
USP 1072 Disinfectants and Aseptics PDF Disinfectant Validation Usp 1072 Organisms dried onto a stainless steel surface. Usp chapter disinfectants and antiseptics. The united states pharmacopoeia (usp) chapter 1072> [7] provides further advice on efficacy testing for the pharmaceutical sector, stating “to demonstrate the efficacy. Disinfectants applied on potential product contact surfaces are typically removed with 70% alcohol wipes. These are the appropriate acceptance criteria for disinfectant efficacy. The requirements. Disinfectant Validation Usp 1072.
From dokumen.tips
(DOC) Disinfectant Efficacy Validation DOKUMEN.TIPS Disinfectant Validation Usp 1072 Disinfectants applied on potential product contact surfaces are typically removed with 70% alcohol wipes. The removal of residual disinfectants. Suspension testing evaluates efficacy of disinfectants under defined laboratory conditions. Disinfectant—a chemical or physical agent that destroys or removes vegetative forms of harmful microorganisms when applied to a surface. The requirements for aseptic processing include readily cleanable floors, walls, and ceilings. Disinfectant Validation Usp 1072.
From www.scribd.com
Disinfection Validation PDF Disinfectant Bacillus Disinfectant Validation Usp 1072 Disinfectant—a chemical or physical agent that destroys or removes vegetative forms of harmful microorganisms when applied to a surface. These are the appropriate acceptance criteria for disinfectant efficacy. Usp disinfectants and antiseptics calls for: Disinfectants applied on potential product contact surfaces are typically removed with 70% alcohol wipes. Usp chapter disinfectants and antiseptics. Suspension testing evaluates efficacy of disinfectants under. Disinfectant Validation Usp 1072.
From microbiologyguideritesh.com
Disinfectant Efficacy Test 2023 Microbiology Guidelines Disinfectant Validation Usp 1072 Usp disinfectants and antiseptics calls for: The removal of residual disinfectants. Suspension testing evaluates efficacy of disinfectants under defined laboratory conditions. Disinfectant—a chemical or physical agent that destroys or removes vegetative forms of harmful microorganisms when applied to a surface. The united states pharmacopoeia (usp) chapter 1072> [7] provides further advice on efficacy testing for the pharmaceutical sector, stating “to. Disinfectant Validation Usp 1072.
From www.researchgate.net
(PDF) Commercial Disinfectants During Disinfection Process Validation Disinfectant Validation Usp 1072 Usp chapter disinfectants and antiseptics. Organisms dried onto a stainless steel surface. Disinfectants applied on potential product contact surfaces are typically removed with 70% alcohol wipes. The united states pharmacopoeia (usp) chapter 1072> [7] provides further advice on efficacy testing for the pharmaceutical sector, stating “to demonstrate the efficacy. The removal of residual disinfectants. Disinfectant—a chemical or physical agent that. Disinfectant Validation Usp 1072.
From www.presentationeze.com
Cleanroom Classification Information & current Best Disinfectant Validation Usp 1072 The removal of residual disinfectants. Usp chapter disinfectants and antiseptics. Organisms dried onto a stainless steel surface. Usp disinfectants and antiseptics calls for: These are the appropriate acceptance criteria for disinfectant efficacy. Disinfectant—a chemical or physical agent that destroys or removes vegetative forms of harmful microorganisms when applied to a surface. Suspension testing evaluates efficacy of disinfectants under defined laboratory. Disinfectant Validation Usp 1072.
From www.thomassci.com
STERIS™ Vesta Syde™ SQ 128 Sterile RTU Disinfectant Disinfectant Validation Usp 1072 The removal of residual disinfectants. Disinfectant—a chemical or physical agent that destroys or removes vegetative forms of harmful microorganisms when applied to a surface. The united states pharmacopoeia (usp) chapter 1072> [7] provides further advice on efficacy testing for the pharmaceutical sector, stating “to demonstrate the efficacy. Organisms dried onto a stainless steel surface. These are the appropriate acceptance criteria. Disinfectant Validation Usp 1072.
From www.scribd.com
Disinfectant Validation Protocol PDF Disinfectant Colony Forming Unit Disinfectant Validation Usp 1072 Usp disinfectants and antiseptics calls for: Disinfectants applied on potential product contact surfaces are typically removed with 70% alcohol wipes. Disinfectant—a chemical or physical agent that destroys or removes vegetative forms of harmful microorganisms when applied to a surface. The united states pharmacopoeia (usp) chapter 1072> [7] provides further advice on efficacy testing for the pharmaceutical sector, stating “to demonstrate. Disinfectant Validation Usp 1072.
From www.youtube.com
Disinfectant Validation I Validation of Cleaning Agents Disinfectant Disinfectant Validation Usp 1072 The removal of residual disinfectants. Disinfectant—a chemical or physical agent that destroys or removes vegetative forms of harmful microorganisms when applied to a surface. These are the appropriate acceptance criteria for disinfectant efficacy. Suspension testing evaluates efficacy of disinfectants under defined laboratory conditions. The requirements for aseptic processing include readily cleanable floors, walls, and ceilings that have smooth and nonporous. Disinfectant Validation Usp 1072.
From www.scribd.com
Disinfectant Validation PDF Colony Forming Unit Disinfectant Disinfectant Validation Usp 1072 The united states pharmacopoeia (usp) chapter 1072> [7] provides further advice on efficacy testing for the pharmaceutical sector, stating “to demonstrate the efficacy. Usp disinfectants and antiseptics calls for: Disinfectants applied on potential product contact surfaces are typically removed with 70% alcohol wipes. The removal of residual disinfectants. Disinfectant—a chemical or physical agent that destroys or removes vegetative forms of. Disinfectant Validation Usp 1072.
From stratixlabs.com
ReadyNow™ Disinfectant Qualification — Stratix Labs Disinfectant Validation Usp 1072 Usp disinfectants and antiseptics calls for: Organisms dried onto a stainless steel surface. The united states pharmacopoeia (usp) chapter 1072> [7] provides further advice on efficacy testing for the pharmaceutical sector, stating “to demonstrate the efficacy. Usp chapter disinfectants and antiseptics. The removal of residual disinfectants. Suspension testing evaluates efficacy of disinfectants under defined laboratory conditions. Disinfectants applied on potential. Disinfectant Validation Usp 1072.
From www.scribd.com
ISPE SingaporeDisinfectant Validation and Efficacy Testing PDF Disinfectant Validation Usp 1072 The removal of residual disinfectants. Usp chapter disinfectants and antiseptics. Disinfectants applied on potential product contact surfaces are typically removed with 70% alcohol wipes. The united states pharmacopoeia (usp) chapter 1072> [7] provides further advice on efficacy testing for the pharmaceutical sector, stating “to demonstrate the efficacy. The requirements for aseptic processing include readily cleanable floors, walls, and ceilings that. Disinfectant Validation Usp 1072.
From www.cirs-group.com
Validation of Cleaning, Disinfection, Sterilization Validation of Disinfectant Validation Usp 1072 Usp chapter disinfectants and antiseptics. Organisms dried onto a stainless steel surface. The requirements for aseptic processing include readily cleanable floors, walls, and ceilings that have smooth and nonporous surfaces;. The united states pharmacopoeia (usp) chapter 1072> [7] provides further advice on efficacy testing for the pharmaceutical sector, stating “to demonstrate the efficacy. Suspension testing evaluates efficacy of disinfectants under. Disinfectant Validation Usp 1072.
From www.m-pharmainfo.com
SOP for Disinfectant Validation Disinfectant Validation Usp 1072 Suspension testing evaluates efficacy of disinfectants under defined laboratory conditions. Disinfectants applied on potential product contact surfaces are typically removed with 70% alcohol wipes. Disinfectant—a chemical or physical agent that destroys or removes vegetative forms of harmful microorganisms when applied to a surface. Usp disinfectants and antiseptics calls for: Organisms dried onto a stainless steel surface. The requirements for aseptic. Disinfectant Validation Usp 1072.
From www.scribd.com
Disinfectant Validation Procedure Microbiology PDF Disinfectant Disinfectant Validation Usp 1072 Usp disinfectants and antiseptics calls for: These are the appropriate acceptance criteria for disinfectant efficacy. Suspension testing evaluates efficacy of disinfectants under defined laboratory conditions. Disinfectant—a chemical or physical agent that destroys or removes vegetative forms of harmful microorganisms when applied to a surface. The united states pharmacopoeia (usp) chapter 1072> [7] provides further advice on efficacy testing for the. Disinfectant Validation Usp 1072.