Solution Reaction Endothermic Or Exothermic . Mix hcl h c l and naoh n a o h together (reactants are at the same temperature) in a calorimeter. The temperature increases, and the book says it's an exothermic reaction. An endothermic reaction occurs when the temperature of an isolated system decreases while the surroundings of a non. Includes kit list and safety instructions. Decide whether various reactions are exothermic or endothermic by measuring temperature change in this practical. Combustion is an example of an exothermic reaction. Endothermic reactions absorb energy from their surroundings, because the products are higher in energy than the reactants. Endothermic and exothermic reactions are chemical reactions that absorb and release heat, respectively. Use bond dissociation energies to calculate enthalpy change or heat of reaction. Determine if a chemical process is exothermic or. Photosynthesis is a good example of an endothermic reaction.
from pediaa.com
An endothermic reaction occurs when the temperature of an isolated system decreases while the surroundings of a non. Combustion is an example of an exothermic reaction. Endothermic reactions absorb energy from their surroundings, because the products are higher in energy than the reactants. Mix hcl h c l and naoh n a o h together (reactants are at the same temperature) in a calorimeter. Decide whether various reactions are exothermic or endothermic by measuring temperature change in this practical. Endothermic and exothermic reactions are chemical reactions that absorb and release heat, respectively. Includes kit list and safety instructions. The temperature increases, and the book says it's an exothermic reaction. Photosynthesis is a good example of an endothermic reaction. Use bond dissociation energies to calculate enthalpy change or heat of reaction.
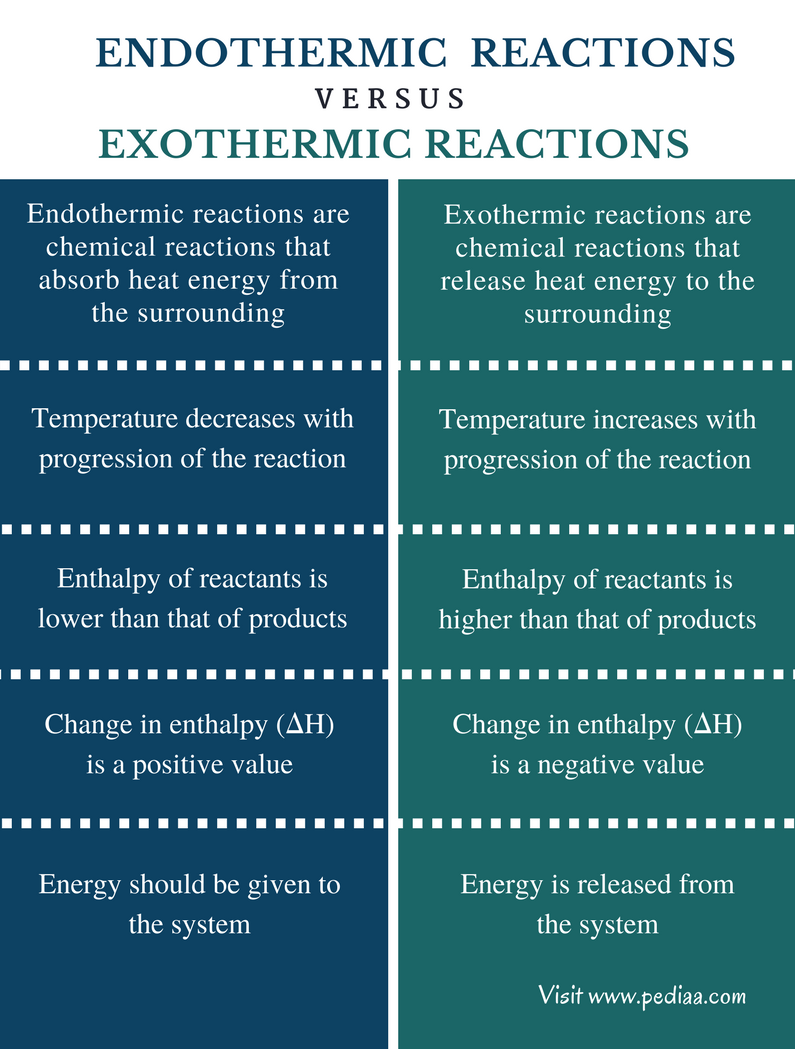
Difference Between Endothermic and Exothermic Reactions Definition
Solution Reaction Endothermic Or Exothermic Combustion is an example of an exothermic reaction. An endothermic reaction occurs when the temperature of an isolated system decreases while the surroundings of a non. Decide whether various reactions are exothermic or endothermic by measuring temperature change in this practical. Determine if a chemical process is exothermic or. The temperature increases, and the book says it's an exothermic reaction. Photosynthesis is a good example of an endothermic reaction. Endothermic reactions absorb energy from their surroundings, because the products are higher in energy than the reactants. Combustion is an example of an exothermic reaction. Mix hcl h c l and naoh n a o h together (reactants are at the same temperature) in a calorimeter. Use bond dissociation energies to calculate enthalpy change or heat of reaction. Includes kit list and safety instructions. Endothermic and exothermic reactions are chemical reactions that absorb and release heat, respectively.
From ar.inspiredpencil.com
Endothermic And Exothermic Reaction Examples Solution Reaction Endothermic Or Exothermic An endothermic reaction occurs when the temperature of an isolated system decreases while the surroundings of a non. Photosynthesis is a good example of an endothermic reaction. Endothermic reactions absorb energy from their surroundings, because the products are higher in energy than the reactants. Mix hcl h c l and naoh n a o h together (reactants are at the. Solution Reaction Endothermic Or Exothermic.
From byjus.com
Difference Between Endothermic and Exothermic Reactions Chemistry Solution Reaction Endothermic Or Exothermic Combustion is an example of an exothermic reaction. Includes kit list and safety instructions. Mix hcl h c l and naoh n a o h together (reactants are at the same temperature) in a calorimeter. Endothermic and exothermic reactions are chemical reactions that absorb and release heat, respectively. Photosynthesis is a good example of an endothermic reaction. Endothermic reactions absorb. Solution Reaction Endothermic Or Exothermic.
From revisechemistry.uk
Exothermic and Endothermic Reactions AQA C5 revisechemistry.uk Solution Reaction Endothermic Or Exothermic Determine if a chemical process is exothermic or. An endothermic reaction occurs when the temperature of an isolated system decreases while the surroundings of a non. Use bond dissociation energies to calculate enthalpy change or heat of reaction. Photosynthesis is a good example of an endothermic reaction. Combustion is an example of an exothermic reaction. Endothermic and exothermic reactions are. Solution Reaction Endothermic Or Exothermic.
From www.coursehero.com
[Solved] Classify the reaction endothermic or exothermic and give the Solution Reaction Endothermic Or Exothermic Determine if a chemical process is exothermic or. Endothermic and exothermic reactions are chemical reactions that absorb and release heat, respectively. An endothermic reaction occurs when the temperature of an isolated system decreases while the surroundings of a non. Decide whether various reactions are exothermic or endothermic by measuring temperature change in this practical. Mix hcl h c l and. Solution Reaction Endothermic Or Exothermic.
From www.studypool.com
SOLUTION Exothermic and endothermic reactions Studypool Solution Reaction Endothermic Or Exothermic Includes kit list and safety instructions. Endothermic reactions absorb energy from their surroundings, because the products are higher in energy than the reactants. The temperature increases, and the book says it's an exothermic reaction. Endothermic and exothermic reactions are chemical reactions that absorb and release heat, respectively. Mix hcl h c l and naoh n a o h together (reactants. Solution Reaction Endothermic Or Exothermic.
From www.pinterest.com
Endothermic and Exothermic Reactions Lab ⋆ Exothermic Solution Reaction Endothermic Or Exothermic Photosynthesis is a good example of an endothermic reaction. Combustion is an example of an exothermic reaction. Endothermic and exothermic reactions are chemical reactions that absorb and release heat, respectively. Use bond dissociation energies to calculate enthalpy change or heat of reaction. An endothermic reaction occurs when the temperature of an isolated system decreases while the surroundings of a non.. Solution Reaction Endothermic Or Exothermic.
From www.slideserve.com
PPT Endothermic & Exothermic Reactions PowerPoint Presentation ID Solution Reaction Endothermic Or Exothermic Photosynthesis is a good example of an endothermic reaction. Endothermic and exothermic reactions are chemical reactions that absorb and release heat, respectively. Determine if a chemical process is exothermic or. Mix hcl h c l and naoh n a o h together (reactants are at the same temperature) in a calorimeter. Decide whether various reactions are exothermic or endothermic by. Solution Reaction Endothermic Or Exothermic.
From classnotes123.com
What does one mean by exothermic and endothermic reactions? Give Solution Reaction Endothermic Or Exothermic Mix hcl h c l and naoh n a o h together (reactants are at the same temperature) in a calorimeter. Use bond dissociation energies to calculate enthalpy change or heat of reaction. Endothermic reactions absorb energy from their surroundings, because the products are higher in energy than the reactants. Includes kit list and safety instructions. An endothermic reaction occurs. Solution Reaction Endothermic Or Exothermic.
From www.studypool.com
SOLUTION Exothermic and endothermic reaction Studypool Solution Reaction Endothermic Or Exothermic Mix hcl h c l and naoh n a o h together (reactants are at the same temperature) in a calorimeter. Endothermic reactions absorb energy from their surroundings, because the products are higher in energy than the reactants. Decide whether various reactions are exothermic or endothermic by measuring temperature change in this practical. Determine if a chemical process is exothermic. Solution Reaction Endothermic Or Exothermic.
From www.studypool.com
SOLUTION Endothermic and exothermic reactions Studypool Solution Reaction Endothermic Or Exothermic Mix hcl h c l and naoh n a o h together (reactants are at the same temperature) in a calorimeter. Endothermic reactions absorb energy from their surroundings, because the products are higher in energy than the reactants. Combustion is an example of an exothermic reaction. An endothermic reaction occurs when the temperature of an isolated system decreases while the. Solution Reaction Endothermic Or Exothermic.
From www.studypool.com
SOLUTION Exothermic and endothermic reactions Studypool Solution Reaction Endothermic Or Exothermic Combustion is an example of an exothermic reaction. Decide whether various reactions are exothermic or endothermic by measuring temperature change in this practical. Endothermic reactions absorb energy from their surroundings, because the products are higher in energy than the reactants. Use bond dissociation energies to calculate enthalpy change or heat of reaction. Mix hcl h c l and naoh n. Solution Reaction Endothermic Or Exothermic.
From online-learning-college.com
Exothermic and endothermic reactions Key differences Solution Reaction Endothermic Or Exothermic Use bond dissociation energies to calculate enthalpy change or heat of reaction. Photosynthesis is a good example of an endothermic reaction. Endothermic and exothermic reactions are chemical reactions that absorb and release heat, respectively. Mix hcl h c l and naoh n a o h together (reactants are at the same temperature) in a calorimeter. Combustion is an example of. Solution Reaction Endothermic Or Exothermic.
From www.animalia-life.club
Endothermic And Exothermic Reaction Examples Solution Reaction Endothermic Or Exothermic Determine if a chemical process is exothermic or. Photosynthesis is a good example of an endothermic reaction. Decide whether various reactions are exothermic or endothermic by measuring temperature change in this practical. Endothermic reactions absorb energy from their surroundings, because the products are higher in energy than the reactants. Includes kit list and safety instructions. The temperature increases, and the. Solution Reaction Endothermic Or Exothermic.
From vhmsscience.weebly.com
Endo/Exothermic Reactions VISTA HEIGHTS 8TH GRADE SCIENCE Solution Reaction Endothermic Or Exothermic Photosynthesis is a good example of an endothermic reaction. Use bond dissociation energies to calculate enthalpy change or heat of reaction. Includes kit list and safety instructions. The temperature increases, and the book says it's an exothermic reaction. An endothermic reaction occurs when the temperature of an isolated system decreases while the surroundings of a non. Endothermic reactions absorb energy. Solution Reaction Endothermic Or Exothermic.
From www.studypool.com
SOLUTION Exothermic and endothermic reaction Studypool Solution Reaction Endothermic Or Exothermic Determine if a chemical process is exothermic or. Decide whether various reactions are exothermic or endothermic by measuring temperature change in this practical. The temperature increases, and the book says it's an exothermic reaction. Use bond dissociation energies to calculate enthalpy change or heat of reaction. An endothermic reaction occurs when the temperature of an isolated system decreases while the. Solution Reaction Endothermic Or Exothermic.
From www.tes.com
Endothermic and Exothermic Temperature Changes Edexcel 91 Teaching Solution Reaction Endothermic Or Exothermic Endothermic reactions absorb energy from their surroundings, because the products are higher in energy than the reactants. Use bond dissociation energies to calculate enthalpy change or heat of reaction. The temperature increases, and the book says it's an exothermic reaction. Determine if a chemical process is exothermic or. An endothermic reaction occurs when the temperature of an isolated system decreases. Solution Reaction Endothermic Or Exothermic.
From www.studypool.com
SOLUTION Understanding exothermic and endothermic reactions unveiling Solution Reaction Endothermic Or Exothermic The temperature increases, and the book says it's an exothermic reaction. Decide whether various reactions are exothermic or endothermic by measuring temperature change in this practical. Determine if a chemical process is exothermic or. Photosynthesis is a good example of an endothermic reaction. Mix hcl h c l and naoh n a o h together (reactants are at the same. Solution Reaction Endothermic Or Exothermic.
From www.studypool.com
SOLUTION Exothermic and endothermic reactions Studypool Solution Reaction Endothermic Or Exothermic Endothermic and exothermic reactions are chemical reactions that absorb and release heat, respectively. Mix hcl h c l and naoh n a o h together (reactants are at the same temperature) in a calorimeter. Includes kit list and safety instructions. Combustion is an example of an exothermic reaction. Decide whether various reactions are exothermic or endothermic by measuring temperature change. Solution Reaction Endothermic Or Exothermic.
From nathalianewsdeleon.blogspot.com
Explain the Difference Between Exothermic and Endothermic Reactions Solution Reaction Endothermic Or Exothermic Combustion is an example of an exothermic reaction. Includes kit list and safety instructions. An endothermic reaction occurs when the temperature of an isolated system decreases while the surroundings of a non. Mix hcl h c l and naoh n a o h together (reactants are at the same temperature) in a calorimeter. Use bond dissociation energies to calculate enthalpy. Solution Reaction Endothermic Or Exothermic.
From vhmsscience.weebly.com
Endo/Exothermic Reactions VISTA HEIGHTS 8TH GRADE SCIENCE Solution Reaction Endothermic Or Exothermic Combustion is an example of an exothermic reaction. Use bond dissociation energies to calculate enthalpy change or heat of reaction. Decide whether various reactions are exothermic or endothermic by measuring temperature change in this practical. Mix hcl h c l and naoh n a o h together (reactants are at the same temperature) in a calorimeter. An endothermic reaction occurs. Solution Reaction Endothermic Or Exothermic.
From revisechemistry.uk
Exothermic and Endothermic Reactions AQA C5 revisechemistry.uk Solution Reaction Endothermic Or Exothermic Combustion is an example of an exothermic reaction. Endothermic and exothermic reactions are chemical reactions that absorb and release heat, respectively. An endothermic reaction occurs when the temperature of an isolated system decreases while the surroundings of a non. Photosynthesis is a good example of an endothermic reaction. Decide whether various reactions are exothermic or endothermic by measuring temperature change. Solution Reaction Endothermic Or Exothermic.
From whatisdiffer.com
Difference Between Endothermic And Exothermic Reactions? Solution Reaction Endothermic Or Exothermic Includes kit list and safety instructions. Use bond dissociation energies to calculate enthalpy change or heat of reaction. Endothermic and exothermic reactions are chemical reactions that absorb and release heat, respectively. An endothermic reaction occurs when the temperature of an isolated system decreases while the surroundings of a non. Decide whether various reactions are exothermic or endothermic by measuring temperature. Solution Reaction Endothermic Or Exothermic.
From sciencenotes.org
Endothermic Reactions Definition and Examples Solution Reaction Endothermic Or Exothermic Endothermic and exothermic reactions are chemical reactions that absorb and release heat, respectively. An endothermic reaction occurs when the temperature of an isolated system decreases while the surroundings of a non. Photosynthesis is a good example of an endothermic reaction. Combustion is an example of an exothermic reaction. Determine if a chemical process is exothermic or. Endothermic reactions absorb energy. Solution Reaction Endothermic Or Exothermic.
From slideplayer.com
Chapter 5 Chemical Reactions and Quantities ppt download Solution Reaction Endothermic Or Exothermic Determine if a chemical process is exothermic or. Endothermic reactions absorb energy from their surroundings, because the products are higher in energy than the reactants. The temperature increases, and the book says it's an exothermic reaction. Mix hcl h c l and naoh n a o h together (reactants are at the same temperature) in a calorimeter. Decide whether various. Solution Reaction Endothermic Or Exothermic.
From question.pandai.org
Endothermic and exothermic reactions Solution Reaction Endothermic Or Exothermic Mix hcl h c l and naoh n a o h together (reactants are at the same temperature) in a calorimeter. Decide whether various reactions are exothermic or endothermic by measuring temperature change in this practical. Combustion is an example of an exothermic reaction. Endothermic reactions absorb energy from their surroundings, because the products are higher in energy than the. Solution Reaction Endothermic Or Exothermic.
From eduinput.com
Exothermic ReactionsCharacteristics, Identification, and Examples Solution Reaction Endothermic Or Exothermic Includes kit list and safety instructions. Determine if a chemical process is exothermic or. Use bond dissociation energies to calculate enthalpy change or heat of reaction. Photosynthesis is a good example of an endothermic reaction. Decide whether various reactions are exothermic or endothermic by measuring temperature change in this practical. Mix hcl h c l and naoh n a o. Solution Reaction Endothermic Or Exothermic.
From fixdiagramzoolatrous.z21.web.core.windows.net
Enthalpy Diagram For Endothermic Reaction Solution Reaction Endothermic Or Exothermic The temperature increases, and the book says it's an exothermic reaction. Combustion is an example of an exothermic reaction. Decide whether various reactions are exothermic or endothermic by measuring temperature change in this practical. An endothermic reaction occurs when the temperature of an isolated system decreases while the surroundings of a non. Includes kit list and safety instructions. Use bond. Solution Reaction Endothermic Or Exothermic.
From online-learning-college.com
Energy level diagrams Endothermic & Exothermic reactions Solution Reaction Endothermic Or Exothermic Use bond dissociation energies to calculate enthalpy change or heat of reaction. Determine if a chemical process is exothermic or. An endothermic reaction occurs when the temperature of an isolated system decreases while the surroundings of a non. Combustion is an example of an exothermic reaction. The temperature increases, and the book says it's an exothermic reaction. Includes kit list. Solution Reaction Endothermic Or Exothermic.
From pediaa.com
Difference Between Endothermic and Exothermic Reactions Definition Solution Reaction Endothermic Or Exothermic Combustion is an example of an exothermic reaction. Includes kit list and safety instructions. Photosynthesis is a good example of an endothermic reaction. Use bond dissociation energies to calculate enthalpy change or heat of reaction. Decide whether various reactions are exothermic or endothermic by measuring temperature change in this practical. Endothermic reactions absorb energy from their surroundings, because the products. Solution Reaction Endothermic Or Exothermic.
From www.thoughtco.com
Endothermic and Exothermic Chemical Reactions Solution Reaction Endothermic Or Exothermic An endothermic reaction occurs when the temperature of an isolated system decreases while the surroundings of a non. Endothermic reactions absorb energy from their surroundings, because the products are higher in energy than the reactants. Determine if a chemical process is exothermic or. Includes kit list and safety instructions. Decide whether various reactions are exothermic or endothermic by measuring temperature. Solution Reaction Endothermic Or Exothermic.
From www.studypool.com
SOLUTION Chemistry exothermic endothermic reactions Studypool Solution Reaction Endothermic Or Exothermic Determine if a chemical process is exothermic or. Decide whether various reactions are exothermic or endothermic by measuring temperature change in this practical. Endothermic reactions absorb energy from their surroundings, because the products are higher in energy than the reactants. An endothermic reaction occurs when the temperature of an isolated system decreases while the surroundings of a non. Includes kit. Solution Reaction Endothermic Or Exothermic.
From www.chemistrylearner.com
Endothermic Reaction Definition, Equation, Graph & Examples Solution Reaction Endothermic Or Exothermic The temperature increases, and the book says it's an exothermic reaction. Determine if a chemical process is exothermic or. Decide whether various reactions are exothermic or endothermic by measuring temperature change in this practical. Endothermic reactions absorb energy from their surroundings, because the products are higher in energy than the reactants. Photosynthesis is a good example of an endothermic reaction.. Solution Reaction Endothermic Or Exothermic.
From khatiar1955a.altervista.org
The Difference Between Endothermic and Exothermic Reactions Solution Reaction Endothermic Or Exothermic Includes kit list and safety instructions. An endothermic reaction occurs when the temperature of an isolated system decreases while the surroundings of a non. Determine if a chemical process is exothermic or. Combustion is an example of an exothermic reaction. Use bond dissociation energies to calculate enthalpy change or heat of reaction. Mix hcl h c l and naoh n. Solution Reaction Endothermic Or Exothermic.
From www.studypool.com
SOLUTION Endothermic and exothermic reactions Studypool Solution Reaction Endothermic Or Exothermic Use bond dissociation energies to calculate enthalpy change or heat of reaction. Endothermic reactions absorb energy from their surroundings, because the products are higher in energy than the reactants. Includes kit list and safety instructions. Mix hcl h c l and naoh n a o h together (reactants are at the same temperature) in a calorimeter. The temperature increases, and. Solution Reaction Endothermic Or Exothermic.
From byjus.com
29. EA>0. Is the reaction endothermic or exothermic Solution Reaction Endothermic Or Exothermic Use bond dissociation energies to calculate enthalpy change or heat of reaction. Endothermic reactions absorb energy from their surroundings, because the products are higher in energy than the reactants. Endothermic and exothermic reactions are chemical reactions that absorb and release heat, respectively. An endothermic reaction occurs when the temperature of an isolated system decreases while the surroundings of a non.. Solution Reaction Endothermic Or Exothermic.