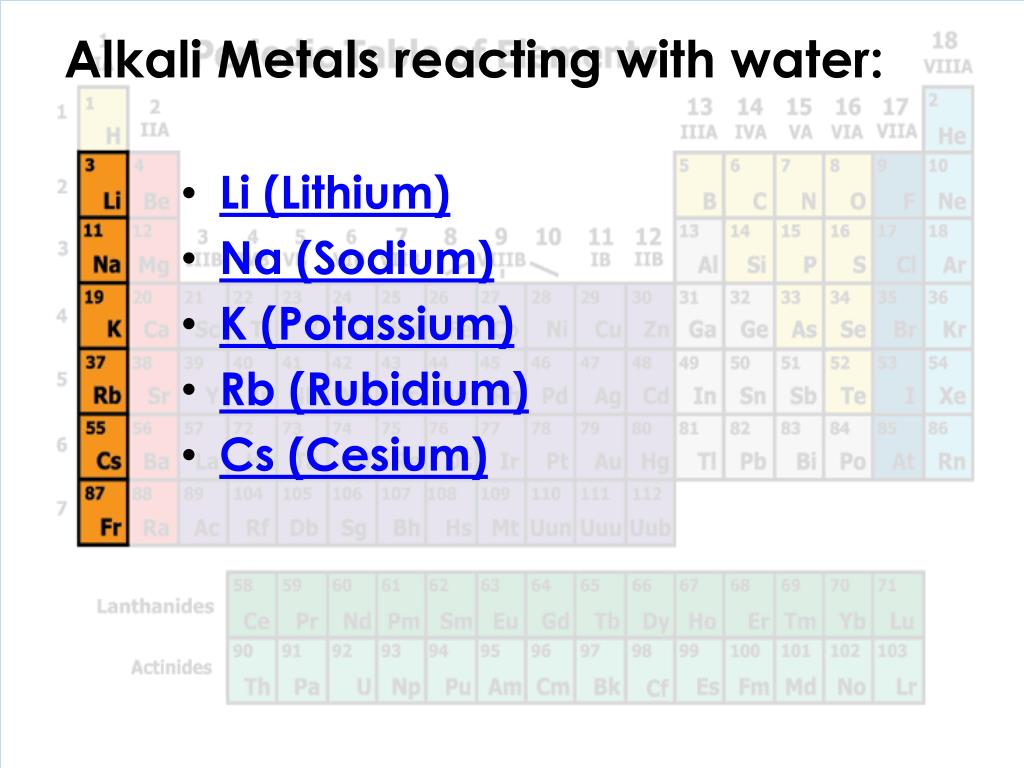
Are Metalloids Reactive With Water . More reactive metals displace less reactive metals from their compounds and. This is a very exothermic reaction (figure \(\pageindex{1}\)). The alkali metals (group 1) are very reactive, readily form ions with a charge of 1+ to form ionic compounds that are usually soluble in water, and react vigorously with water to. 9 rows the reactivity series ranks metals by how readily they react. Alkali metals react with water to produce hydrogen gas and alkali metal hydroxides; The metalloids are boron, silicon,. A series of six elements called the metalloids separate the metals from the nonmetals in the periodic table. The alkali metals (group 1) are very reactive, readily form ions with a charge of 1+ to form ionic compounds that are usually soluble in water,.
from www.slideserve.com
The alkali metals (group 1) are very reactive, readily form ions with a charge of 1+ to form ionic compounds that are usually soluble in water, and react vigorously with water to. A series of six elements called the metalloids separate the metals from the nonmetals in the periodic table. This is a very exothermic reaction (figure \(\pageindex{1}\)). The alkali metals (group 1) are very reactive, readily form ions with a charge of 1+ to form ionic compounds that are usually soluble in water,. More reactive metals displace less reactive metals from their compounds and. Alkali metals react with water to produce hydrogen gas and alkali metal hydroxides; 9 rows the reactivity series ranks metals by how readily they react. The metalloids are boron, silicon,.
PPT Metals. Metalloids. Nonmetals. PowerPoint Presentation, free
Are Metalloids Reactive With Water Alkali metals react with water to produce hydrogen gas and alkali metal hydroxides; Alkali metals react with water to produce hydrogen gas and alkali metal hydroxides; 9 rows the reactivity series ranks metals by how readily they react. This is a very exothermic reaction (figure \(\pageindex{1}\)). The alkali metals (group 1) are very reactive, readily form ions with a charge of 1+ to form ionic compounds that are usually soluble in water,. A series of six elements called the metalloids separate the metals from the nonmetals in the periodic table. The metalloids are boron, silicon,. The alkali metals (group 1) are very reactive, readily form ions with a charge of 1+ to form ionic compounds that are usually soluble in water, and react vigorously with water to. More reactive metals displace less reactive metals from their compounds and.
From www.slideserve.com
PPT Chemical Reactivity and Storage PowerPoint Presentation, free Are Metalloids Reactive With Water The metalloids are boron, silicon,. 9 rows the reactivity series ranks metals by how readily they react. This is a very exothermic reaction (figure \(\pageindex{1}\)). More reactive metals displace less reactive metals from their compounds and. A series of six elements called the metalloids separate the metals from the nonmetals in the periodic table. The alkali metals (group 1) are. Are Metalloids Reactive With Water.
From www.slideserve.com
PPT Metals. Metalloids. Nonmetals. PowerPoint Presentation, free Are Metalloids Reactive With Water A series of six elements called the metalloids separate the metals from the nonmetals in the periodic table. This is a very exothermic reaction (figure \(\pageindex{1}\)). More reactive metals displace less reactive metals from their compounds and. The alkali metals (group 1) are very reactive, readily form ions with a charge of 1+ to form ionic compounds that are usually. Are Metalloids Reactive With Water.
From periodictableguide.com
Periodic table labeled with Metals Nonmetals and Metalloids Are Metalloids Reactive With Water The metalloids are boron, silicon,. Alkali metals react with water to produce hydrogen gas and alkali metal hydroxides; The alkali metals (group 1) are very reactive, readily form ions with a charge of 1+ to form ionic compounds that are usually soluble in water, and react vigorously with water to. A series of six elements called the metalloids separate the. Are Metalloids Reactive With Water.
From slideplayer.com
Mapping the Periodic Table ppt download Are Metalloids Reactive With Water The alkali metals (group 1) are very reactive, readily form ions with a charge of 1+ to form ionic compounds that are usually soluble in water,. The alkali metals (group 1) are very reactive, readily form ions with a charge of 1+ to form ionic compounds that are usually soluble in water, and react vigorously with water to. Alkali metals. Are Metalloids Reactive With Water.
From www.youtube.com
How do metals react with water/Reaction of metals with waterMetals and Are Metalloids Reactive With Water 9 rows the reactivity series ranks metals by how readily they react. The alkali metals (group 1) are very reactive, readily form ions with a charge of 1+ to form ionic compounds that are usually soluble in water,. Alkali metals react with water to produce hydrogen gas and alkali metal hydroxides; The alkali metals (group 1) are very reactive, readily. Are Metalloids Reactive With Water.
From www.researchgate.net
Chemical speciation for metals and metalloids in the water at study Are Metalloids Reactive With Water The alkali metals (group 1) are very reactive, readily form ions with a charge of 1+ to form ionic compounds that are usually soluble in water,. More reactive metals displace less reactive metals from their compounds and. 9 rows the reactivity series ranks metals by how readily they react. This is a very exothermic reaction (figure \(\pageindex{1}\)). The metalloids are. Are Metalloids Reactive With Water.
From slideplayer.com
Atoms, Molecules, and Ions ppt download Are Metalloids Reactive With Water The alkali metals (group 1) are very reactive, readily form ions with a charge of 1+ to form ionic compounds that are usually soluble in water,. A series of six elements called the metalloids separate the metals from the nonmetals in the periodic table. This is a very exothermic reaction (figure \(\pageindex{1}\)). 9 rows the reactivity series ranks metals by. Are Metalloids Reactive With Water.
From www.hanlin.com
EDEXCEL IGCSE CHEMISTRY DOUBLE SCIENCE 复习笔记:2.4.1 Metals Reacting with Are Metalloids Reactive With Water The alkali metals (group 1) are very reactive, readily form ions with a charge of 1+ to form ionic compounds that are usually soluble in water, and react vigorously with water to. A series of six elements called the metalloids separate the metals from the nonmetals in the periodic table. The alkali metals (group 1) are very reactive, readily form. Are Metalloids Reactive With Water.
From thechemistrynotes.com
Metalloids Definition, Properties, Uses, and Applications Are Metalloids Reactive With Water The alkali metals (group 1) are very reactive, readily form ions with a charge of 1+ to form ionic compounds that are usually soluble in water, and react vigorously with water to. 9 rows the reactivity series ranks metals by how readily they react. More reactive metals displace less reactive metals from their compounds and. A series of six elements. Are Metalloids Reactive With Water.
From abhyasonline.in
Concept Explanation Are Metalloids Reactive With Water This is a very exothermic reaction (figure \(\pageindex{1}\)). Alkali metals react with water to produce hydrogen gas and alkali metal hydroxides; The alkali metals (group 1) are very reactive, readily form ions with a charge of 1+ to form ionic compounds that are usually soluble in water,. The metalloids are boron, silicon,. A series of six elements called the metalloids. Are Metalloids Reactive With Water.
From slideplayer.com
Check Unit 3 NOTES Lesson 1. Check Unit 3 NOTES Lesson ppt download Are Metalloids Reactive With Water A series of six elements called the metalloids separate the metals from the nonmetals in the periodic table. More reactive metals displace less reactive metals from their compounds and. Alkali metals react with water to produce hydrogen gas and alkali metal hydroxides; The alkali metals (group 1) are very reactive, readily form ions with a charge of 1+ to form. Are Metalloids Reactive With Water.
From byjus.com
5. Lithium metal reacts with water gently while Na metal reacts with Are Metalloids Reactive With Water This is a very exothermic reaction (figure \(\pageindex{1}\)). The alkali metals (group 1) are very reactive, readily form ions with a charge of 1+ to form ionic compounds that are usually soluble in water, and react vigorously with water to. The metalloids are boron, silicon,. The alkali metals (group 1) are very reactive, readily form ions with a charge of. Are Metalloids Reactive With Water.
From www.researchgate.net
Chemical speciation for metals and metalloids in the water at study Are Metalloids Reactive With Water A series of six elements called the metalloids separate the metals from the nonmetals in the periodic table. The metalloids are boron, silicon,. More reactive metals displace less reactive metals from their compounds and. The alkali metals (group 1) are very reactive, readily form ions with a charge of 1+ to form ionic compounds that are usually soluble in water,.. Are Metalloids Reactive With Water.
From www.youtube.com
Reaction of Metallic oxides YouTube Are Metalloids Reactive With Water The metalloids are boron, silicon,. More reactive metals displace less reactive metals from their compounds and. The alkali metals (group 1) are very reactive, readily form ions with a charge of 1+ to form ionic compounds that are usually soluble in water,. The alkali metals (group 1) are very reactive, readily form ions with a charge of 1+ to form. Are Metalloids Reactive With Water.
From www.adda247.com
What are Metalloids? Definition, Properties and Example Are Metalloids Reactive With Water The metalloids are boron, silicon,. 9 rows the reactivity series ranks metals by how readily they react. The alkali metals (group 1) are very reactive, readily form ions with a charge of 1+ to form ionic compounds that are usually soluble in water,. This is a very exothermic reaction (figure \(\pageindex{1}\)). The alkali metals (group 1) are very reactive, readily. Are Metalloids Reactive With Water.
From www.slideserve.com
PPT Properties of Metals, Nonmetals, and Metalloids PowerPoint Are Metalloids Reactive With Water The alkali metals (group 1) are very reactive, readily form ions with a charge of 1+ to form ionic compounds that are usually soluble in water, and react vigorously with water to. This is a very exothermic reaction (figure \(\pageindex{1}\)). The alkali metals (group 1) are very reactive, readily form ions with a charge of 1+ to form ionic compounds. Are Metalloids Reactive With Water.
From www.youtube.com
Metalloids Reaction of Metalloid Chemistry YouTube Are Metalloids Reactive With Water The metalloids are boron, silicon,. A series of six elements called the metalloids separate the metals from the nonmetals in the periodic table. The alkali metals (group 1) are very reactive, readily form ions with a charge of 1+ to form ionic compounds that are usually soluble in water, and react vigorously with water to. The alkali metals (group 1). Are Metalloids Reactive With Water.
From www.teachoo.com
Reaction of Metals and Nonmetals with Water with examples Teachoo Are Metalloids Reactive With Water This is a very exothermic reaction (figure \(\pageindex{1}\)). The alkali metals (group 1) are very reactive, readily form ions with a charge of 1+ to form ionic compounds that are usually soluble in water,. A series of six elements called the metalloids separate the metals from the nonmetals in the periodic table. The alkali metals (group 1) are very reactive,. Are Metalloids Reactive With Water.
From animalia-life.club
Periodic Table With Metals Nonmetals And Metalloids Are Metalloids Reactive With Water This is a very exothermic reaction (figure \(\pageindex{1}\)). Alkali metals react with water to produce hydrogen gas and alkali metal hydroxides; The metalloids are boron, silicon,. The alkali metals (group 1) are very reactive, readily form ions with a charge of 1+ to form ionic compounds that are usually soluble in water, and react vigorously with water to. 9 rows. Are Metalloids Reactive With Water.
From www.researchgate.net
Metal and metalloid concentrations of water and sediment samples Are Metalloids Reactive With Water The alkali metals (group 1) are very reactive, readily form ions with a charge of 1+ to form ionic compounds that are usually soluble in water,. This is a very exothermic reaction (figure \(\pageindex{1}\)). The alkali metals (group 1) are very reactive, readily form ions with a charge of 1+ to form ionic compounds that are usually soluble in water,. Are Metalloids Reactive With Water.
From www.youtube.com
Reaction of metals with water Class 10 Chemistry ICSE Board Are Metalloids Reactive With Water A series of six elements called the metalloids separate the metals from the nonmetals in the periodic table. This is a very exothermic reaction (figure \(\pageindex{1}\)). The metalloids are boron, silicon,. More reactive metals displace less reactive metals from their compounds and. The alkali metals (group 1) are very reactive, readily form ions with a charge of 1+ to form. Are Metalloids Reactive With Water.
From kunduz.com
[ANSWERED] We know that metal react with water to gi... Physical Are Metalloids Reactive With Water The metalloids are boron, silicon,. This is a very exothermic reaction (figure \(\pageindex{1}\)). A series of six elements called the metalloids separate the metals from the nonmetals in the periodic table. 9 rows the reactivity series ranks metals by how readily they react. The alkali metals (group 1) are very reactive, readily form ions with a charge of 1+ to. Are Metalloids Reactive With Water.
From www.youtube.com
Reactions of Metallic Oxides with Water Chemistry Class 10 Embibe Are Metalloids Reactive With Water The alkali metals (group 1) are very reactive, readily form ions with a charge of 1+ to form ionic compounds that are usually soluble in water, and react vigorously with water to. 9 rows the reactivity series ranks metals by how readily they react. The metalloids are boron, silicon,. This is a very exothermic reaction (figure \(\pageindex{1}\)). The alkali metals. Are Metalloids Reactive With Water.
From bestweddingblog47.blogspot.com
Which Group Of Metals Is The Most Reactive Are Metalloids Reactive With Water 9 rows the reactivity series ranks metals by how readily they react. More reactive metals displace less reactive metals from their compounds and. A series of six elements called the metalloids separate the metals from the nonmetals in the periodic table. Alkali metals react with water to produce hydrogen gas and alkali metal hydroxides; The alkali metals (group 1) are. Are Metalloids Reactive With Water.
From www.researchgate.net
Concentrations of metal/metalloids in water (a, b) (µg/l) and sediment Are Metalloids Reactive With Water The metalloids are boron, silicon,. A series of six elements called the metalloids separate the metals from the nonmetals in the periodic table. Alkali metals react with water to produce hydrogen gas and alkali metal hydroxides; This is a very exothermic reaction (figure \(\pageindex{1}\)). The alkali metals (group 1) are very reactive, readily form ions with a charge of 1+. Are Metalloids Reactive With Water.
From askfilo.com
Metals react with water and produce a metal oxide and hydrogen gas. Metal.. Are Metalloids Reactive With Water A series of six elements called the metalloids separate the metals from the nonmetals in the periodic table. The metalloids are boron, silicon,. The alkali metals (group 1) are very reactive, readily form ions with a charge of 1+ to form ionic compounds that are usually soluble in water, and react vigorously with water to. This is a very exothermic. Are Metalloids Reactive With Water.
From www.xometry.com
Metalloids Properties and Uses Xometry Are Metalloids Reactive With Water More reactive metals displace less reactive metals from their compounds and. This is a very exothermic reaction (figure \(\pageindex{1}\)). The metalloids are boron, silicon,. A series of six elements called the metalloids separate the metals from the nonmetals in the periodic table. The alkali metals (group 1) are very reactive, readily form ions with a charge of 1+ to form. Are Metalloids Reactive With Water.
From www.slideserve.com
PPT Metals, Metalloids, and Nonmetals PowerPoint Presentation, free Are Metalloids Reactive With Water The alkali metals (group 1) are very reactive, readily form ions with a charge of 1+ to form ionic compounds that are usually soluble in water,. 9 rows the reactivity series ranks metals by how readily they react. The alkali metals (group 1) are very reactive, readily form ions with a charge of 1+ to form ionic compounds that are. Are Metalloids Reactive With Water.
From www.slideserve.com
PPT Chapter 7 Periodic Properties of the Elements PowerPoint Are Metalloids Reactive With Water The metalloids are boron, silicon,. A series of six elements called the metalloids separate the metals from the nonmetals in the periodic table. This is a very exothermic reaction (figure \(\pageindex{1}\)). Alkali metals react with water to produce hydrogen gas and alkali metal hydroxides; More reactive metals displace less reactive metals from their compounds and. 9 rows the reactivity series. Are Metalloids Reactive With Water.
From www.slideserve.com
PPT Chemical Bonding PowerPoint Presentation, free download ID1992532 Are Metalloids Reactive With Water A series of six elements called the metalloids separate the metals from the nonmetals in the periodic table. 9 rows the reactivity series ranks metals by how readily they react. This is a very exothermic reaction (figure \(\pageindex{1}\)). Alkali metals react with water to produce hydrogen gas and alkali metal hydroxides; More reactive metals displace less reactive metals from their. Are Metalloids Reactive With Water.
From slideplayer.com
Chapter 7 Periodic Properties of the Elements ppt download Are Metalloids Reactive With Water The alkali metals (group 1) are very reactive, readily form ions with a charge of 1+ to form ionic compounds that are usually soluble in water, and react vigorously with water to. A series of six elements called the metalloids separate the metals from the nonmetals in the periodic table. The alkali metals (group 1) are very reactive, readily form. Are Metalloids Reactive With Water.
From slideplayer.com
Periodic Properties of the Elements ppt download Are Metalloids Reactive With Water More reactive metals displace less reactive metals from their compounds and. This is a very exothermic reaction (figure \(\pageindex{1}\)). The metalloids are boron, silicon,. The alkali metals (group 1) are very reactive, readily form ions with a charge of 1+ to form ionic compounds that are usually soluble in water,. Alkali metals react with water to produce hydrogen gas and. Are Metalloids Reactive With Water.
From slideplayer.com
PERIODIC TABLE. ppt download Are Metalloids Reactive With Water A series of six elements called the metalloids separate the metals from the nonmetals in the periodic table. The metalloids are boron, silicon,. The alkali metals (group 1) are very reactive, readily form ions with a charge of 1+ to form ionic compounds that are usually soluble in water,. 9 rows the reactivity series ranks metals by how readily they. Are Metalloids Reactive With Water.
From www.youtube.com
Reactions Of Metals With Water Reactions Chemistry FuseSchool Are Metalloids Reactive With Water More reactive metals displace less reactive metals from their compounds and. The metalloids are boron, silicon,. A series of six elements called the metalloids separate the metals from the nonmetals in the periodic table. Alkali metals react with water to produce hydrogen gas and alkali metal hydroxides; This is a very exothermic reaction (figure \(\pageindex{1}\)). The alkali metals (group 1). Are Metalloids Reactive With Water.
From www.chemistrylearner.com
Reactivity Series Definition and Chart Are Metalloids Reactive With Water The metalloids are boron, silicon,. Alkali metals react with water to produce hydrogen gas and alkali metal hydroxides; The alkali metals (group 1) are very reactive, readily form ions with a charge of 1+ to form ionic compounds that are usually soluble in water,. More reactive metals displace less reactive metals from their compounds and. The alkali metals (group 1). Are Metalloids Reactive With Water.