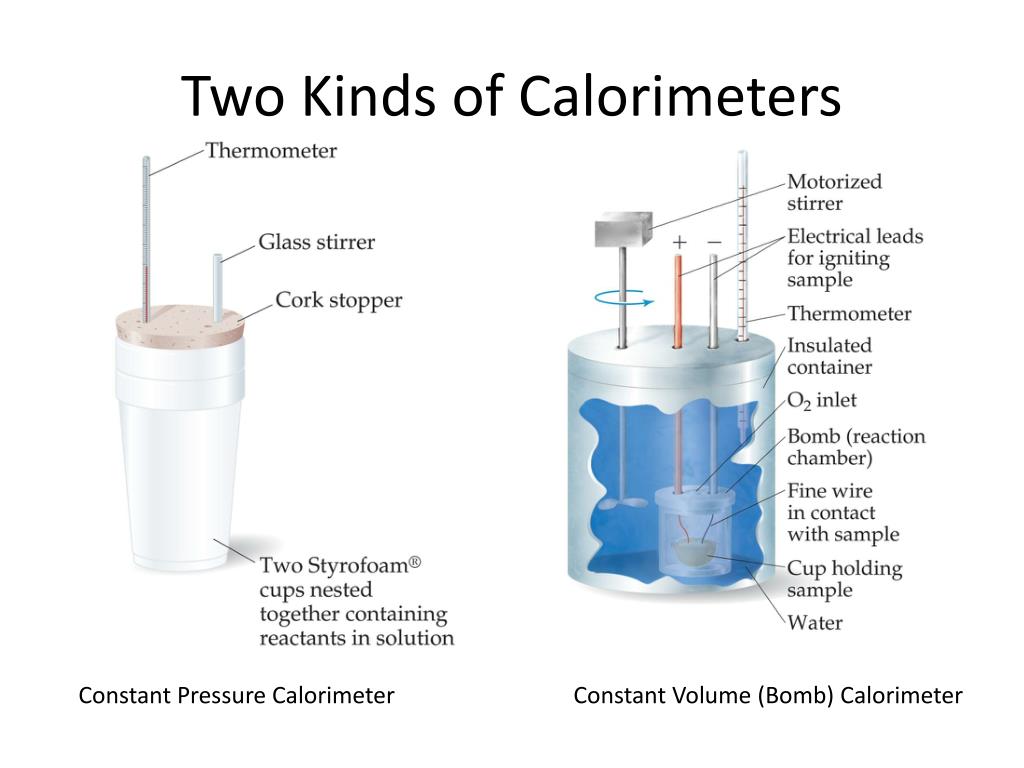
Calorimeter Solution Definition . Calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends on the amount of heat released or absorbed and on. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. For example, when an exothermic. If we run an exothermic reaction in solution in a calorimeter, the heat produced by the reaction is trapped in the calorimeter and increases the temperature of the solution. Apply the first law of thermodynamics to calorimetry. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. For example, when an exothermic. For example, when an exothermic. For example, when an exothermic.
from www.slideserve.com
For example, when an exothermic. Calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends on the amount of heat released or absorbed and on. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. For example, when an exothermic. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. For example, when an exothermic. If we run an exothermic reaction in solution in a calorimeter, the heat produced by the reaction is trapped in the calorimeter and increases the temperature of the solution. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. For example, when an exothermic.
PPT Calorimetry PowerPoint Presentation, free download ID3850751
Calorimeter Solution Definition For example, when an exothermic. Calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends on the amount of heat released or absorbed and on. Apply the first law of thermodynamics to calorimetry. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. For example, when an exothermic. For example, when an exothermic. For example, when an exothermic. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. For example, when an exothermic. If we run an exothermic reaction in solution in a calorimeter, the heat produced by the reaction is trapped in the calorimeter and increases the temperature of the solution. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process.
From www.youtube.com
Thermal Properties of Matter Class 11 Physics Calorimetry Principle Calorimeter Solution Definition For example, when an exothermic. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Calorimetry. Calorimeter Solution Definition.
From courses.lumenlearning.com
Calorimetry Chemistry for Majors Calorimeter Solution Definition For example, when an exothermic. If we run an exothermic reaction in solution in a calorimeter, the heat produced by the reaction is trapped in the calorimeter and increases the temperature of the solution. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Compare heat flow from hot to cold. Calorimeter Solution Definition.
From mechasource.blogspot.com
An Introduction To Calorimetry types And Uses , Bomb and Boy,s Gas Calorimeter Solution Definition Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. Calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends on the amount of heat released or absorbed and on. If we run an exothermic reaction in solution in a calorimeter, the heat produced by the reaction is trapped. Calorimeter Solution Definition.
From glossary.periodni.com
Bomb calorimeter Chemistry Dictionary & Glossary Calorimeter Solution Definition Calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends on the amount of heat released or absorbed and on. For example, when an exothermic. For example, when an exothermic. For example, when an exothermic. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. A calorimeter is a. Calorimeter Solution Definition.
From www.britannica.com
Calorimeter Definition, Uses, Diagram, & Facts Britannica Calorimeter Solution Definition Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. Calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends on the amount of heat released or absorbed and on. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process.. Calorimeter Solution Definition.
From porter-yersblogvega.blogspot.com
Heat Capacity of Calorimeter Calorimeter Solution Definition Calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends on the amount of heat released or absorbed and on. For example, when an exothermic. If we run an exothermic reaction in solution in a calorimeter, the heat produced by the reaction is trapped in the calorimeter and increases the temperature of the solution. A. Calorimeter Solution Definition.
From saylordotorg.github.io
Calorimetry Calorimeter Solution Definition A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. For example, when an exothermic. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. A calorimeter is a device used to measure the amount of heat involved in a chemical or. Calorimeter Solution Definition.
From www.chemistrylearner.com
Heat (Enthalpy) of Solution Definition, Formula, & Problems Calorimeter Solution Definition A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. For example, when an exothermic. Apply the first law of thermodynamics to calorimetry. For example, when an exothermic. Calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends on the amount of heat released or absorbed. Calorimeter Solution Definition.
From eduinput.com
CalorimeterDefinition, History, Construction, Types, And Uses Calorimeter Solution Definition For example, when an exothermic. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. Apply the first law of thermodynamics to calorimetry. For example, when an exothermic. A calorimeter is a device used to. Calorimeter Solution Definition.
From grade12uchemistry.weebly.com
Calorimetry Grade12UChemistry Calorimeter Solution Definition For example, when an exothermic. Apply the first law of thermodynamics to calorimetry. Calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends on the amount of heat released or absorbed and on. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. For example, when. Calorimeter Solution Definition.
From quizdbbarefooted.z21.web.core.windows.net
How To Calculate Calorimeter Calorimeter Solution Definition Apply the first law of thermodynamics to calorimetry. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. If we run an exothermic reaction in solution in a calorimeter, the heat produced. Calorimeter Solution Definition.
From courses.lumenlearning.com
5.2 Calorimetry Chemistry Calorimeter Solution Definition For example, when an exothermic. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. For example, when an exothermic. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. If we run an exothermic reaction in solution in a calorimeter, the. Calorimeter Solution Definition.
From www.collegesearch.in
Principle of Calorimetry Definition, Formula, Principle, Types Calorimeter Solution Definition Apply the first law of thermodynamics to calorimetry. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. For example, when an exothermic. For example, when an exothermic. Compare heat flow from. Calorimeter Solution Definition.
From www.studypool.com
SOLUTION Chemistry cit u calorimetry definition examples sample Calorimeter Solution Definition For example, when an exothermic. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. For example, when an exothermic. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. For example, when an exothermic. For example, when an exothermic. Calorimetry measures. Calorimeter Solution Definition.
From dxomoddbu.blob.core.windows.net
How Does Direct Calorimetry Work at Christine Cahill blog Calorimeter Solution Definition For example, when an exothermic. For example, when an exothermic. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. If we run an exothermic reaction in solution in a calorimeter, the heat produced by the reaction is trapped in the calorimeter and increases the temperature of the solution. Calorimetry measures. Calorimeter Solution Definition.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation, free download ID3850751 Calorimeter Solution Definition For example, when an exothermic. Calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends on the amount of heat released or absorbed and on. If we run an exothermic reaction in solution in a calorimeter, the heat produced by the reaction is trapped in the calorimeter and increases the temperature of the solution. A. Calorimeter Solution Definition.
From www.thoughtco.com
Calorimeter Definition in Chemistry Calorimeter Solution Definition For example, when an exothermic. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. For. Calorimeter Solution Definition.
From wisc.pb.unizin.org
5.2 Calorimetry Chemistry Calorimeter Solution Definition For example, when an exothermic. Apply the first law of thermodynamics to calorimetry. If we run an exothermic reaction in solution in a calorimeter, the heat produced by the reaction is trapped in the calorimeter and increases the temperature of the solution. For example, when an exothermic. A calorimeter is a device used to measure the amount of heat involved. Calorimeter Solution Definition.
From serc.carleton.edu
Calorimetry Calorimeter Solution Definition For example, when an exothermic. For example, when an exothermic. Calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends on the amount of heat released or absorbed and on. For example, when an exothermic. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Compare. Calorimeter Solution Definition.
From courses.lumenlearning.com
Calorimetry Chemistry I Calorimeter Solution Definition If we run an exothermic reaction in solution in a calorimeter, the heat produced by the reaction is trapped in the calorimeter and increases the temperature of the solution. Apply the first law of thermodynamics to calorimetry. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. A calorimeter is a. Calorimeter Solution Definition.
From exollkmhz.blob.core.windows.net
Define Adiabatic Calorimeter at Alma Martin blog Calorimeter Solution Definition A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. For example, when an exothermic. For example, when an exothermic. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. A calorimeter is a device used to measure the amount of heat. Calorimeter Solution Definition.
From www.studypool.com
SOLUTION Chemistry cit u calorimetry definition examples sample Calorimeter Solution Definition If we run an exothermic reaction in solution in a calorimeter, the heat produced by the reaction is trapped in the calorimeter and increases the temperature of the solution. For example, when an exothermic. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Calorimetry measures enthalpy changes during chemical processes,. Calorimeter Solution Definition.
From www.slideshare.net
Lesson Enthalpy and Calorimetry Calorimeter Solution Definition A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. Apply the first law of thermodynamics to calorimetry. If we run an exothermic reaction in solution in a calorimeter, the heat produced by the reaction. Calorimeter Solution Definition.
From physique.ensc-rennes.fr
TP calorimetry Calorimeter Solution Definition For example, when an exothermic. Apply the first law of thermodynamics to calorimetry. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. If we run an exothermic reaction in solution in a calorimeter, the. Calorimeter Solution Definition.
From www.chegg.com
Solved In the laboratory a "coffee cup" calorimeter, or Calorimeter Solution Definition A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Apply the first law of thermodynamics to calorimetry. If we run an exothermic reaction in solution in a calorimeter, the heat produced by the reaction is trapped in the calorimeter and increases the temperature of the solution. A calorimeter is a. Calorimeter Solution Definition.
From www.pinterest.com
ConstantPressure Calorimetery "coffeecup" calorimeter often used to Calorimeter Solution Definition For example, when an exothermic. For example, when an exothermic. If we run an exothermic reaction in solution in a calorimeter, the heat produced by the reaction is trapped in the calorimeter and increases the temperature of the solution. For example, when an exothermic. A calorimeter is a device used to measure the amount of heat involved in a chemical. Calorimeter Solution Definition.
From users.highland.edu
Calorimetry Calorimeter Solution Definition For example, when an exothermic. For example, when an exothermic. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Apply the first law of thermodynamics to calorimetry. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. For example, when an. Calorimeter Solution Definition.
From www.studypool.com
SOLUTION Chemistry cit u calorimetry definition examples sample Calorimeter Solution Definition For example, when an exothermic. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Apply the first law of thermodynamics to calorimetry. A calorimeter is a device used to measure the. Calorimeter Solution Definition.
From www.slideserve.com
PPT Chapter 5 PowerPoint Presentation, free download ID197266 Calorimeter Solution Definition For example, when an exothermic. Calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends on the amount of heat released or absorbed and on. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. If we run an exothermic reaction in solution in a calorimeter,. Calorimeter Solution Definition.
From study.com
Bomb Calorimeter Uses, Equations & Examples Lesson Calorimeter Solution Definition Apply the first law of thermodynamics to calorimetry. For example, when an exothermic. Calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends on the amount of heat released or absorbed and on. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. A calorimeter is a device used. Calorimeter Solution Definition.
From www.youtube.com
BASIC PRINCIPLE OF CALORIMETRY YouTube Calorimeter Solution Definition If we run an exothermic reaction in solution in a calorimeter, the heat produced by the reaction is trapped in the calorimeter and increases the temperature of the solution. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Calorimetry measures enthalpy changes during chemical processes, where the magnitude of the. Calorimeter Solution Definition.
From fyohqoqgp.blob.core.windows.net
Calorimeter Formula In Physics at Caroline Graig blog Calorimeter Solution Definition Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. For example, when an exothermic. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Calorimetry. Calorimeter Solution Definition.
From faqguide.co
What does a calorimeter do? Explained by FAQGuide Calorimeter Solution Definition If we run an exothermic reaction in solution in a calorimeter, the heat produced by the reaction is trapped in the calorimeter and increases the temperature of the solution. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. For example, when an exothermic. A calorimeter is a device used to. Calorimeter Solution Definition.
From klaxzllai.blob.core.windows.net
Calorimetry Equation Examples at Grace Smallwood blog Calorimeter Solution Definition A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. If we run an exothermic reaction in solution in a calorimeter, the heat produced by the reaction is trapped in the calorimeter. Calorimeter Solution Definition.
From www.scienceabc.com
Molar Heat Capacity Definition, Formula, Equation, Calculation Calorimeter Solution Definition For example, when an exothermic. If we run an exothermic reaction in solution in a calorimeter, the heat produced by the reaction is trapped in the calorimeter and increases the temperature of the solution. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Calorimetry measures enthalpy changes during chemical processes,. Calorimeter Solution Definition.