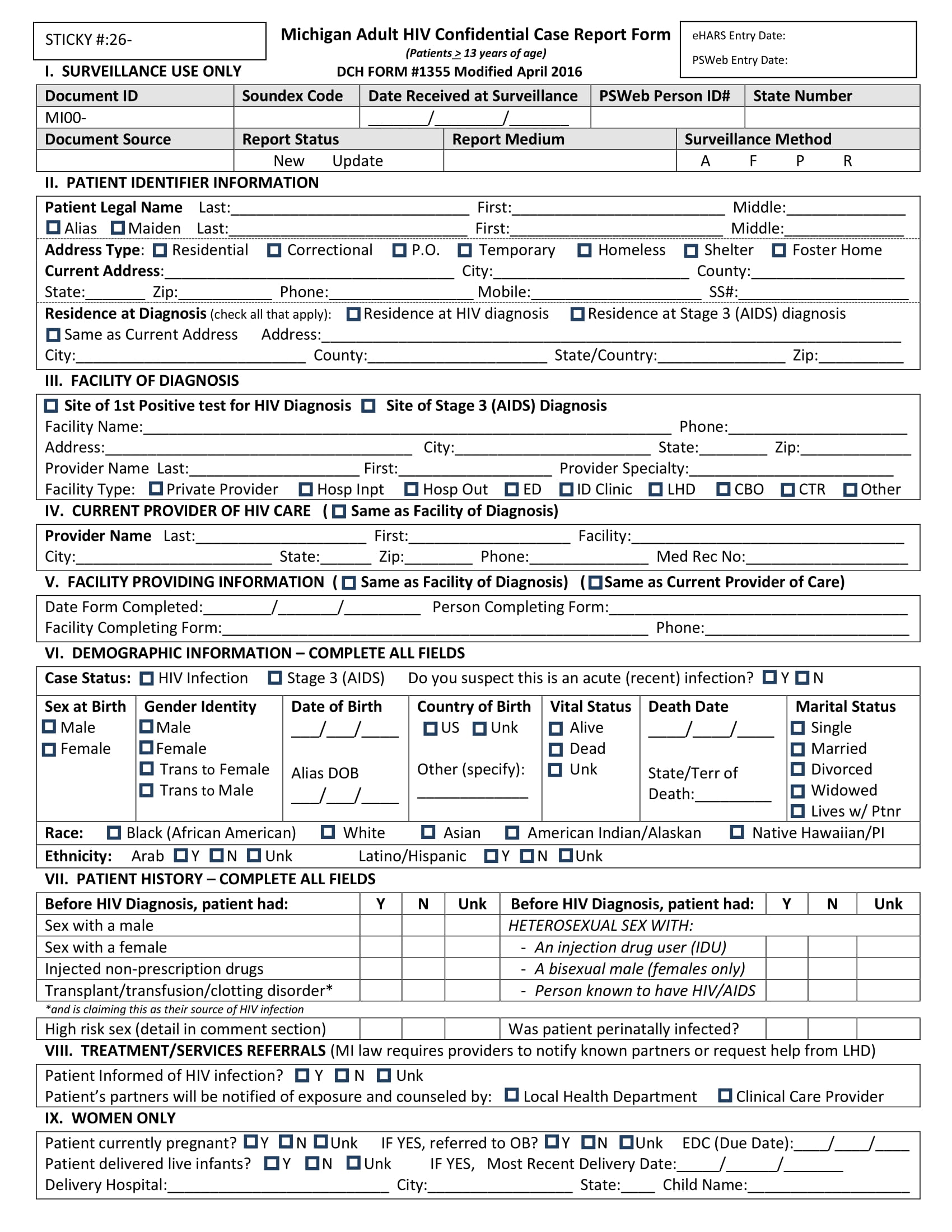
Case Report Form Pdf . Definitions case report form (crf) form on which individual participant data required by the study protocol are recorded. State the obvious start by asking yourself: Further information is available online on rates and how. It may be a paper. Case report forms (crfs) are used to collect data in clinical research. To be considered to be not of childbearing potential, the subject must be postmenopausal for at least 2 years; Clinical findings and outcome step 1: You or your institution must be a fellow of bmj case reports in order to submit. Case report form development represents a significant part of the clinical trial process and can affect study. Case report form (crf) is a specialized document in clinical research. 1.2 the case report form (crf) is a data collection tool used to capture the required data, as defined by the protocol, for each individual subject. Writing the core of your case report:
from www.sampleforms.com
1.2 the case report form (crf) is a data collection tool used to capture the required data, as defined by the protocol, for each individual subject. Case report forms (crfs) are used to collect data in clinical research. Case report form (crf) is a specialized document in clinical research. To be considered to be not of childbearing potential, the subject must be postmenopausal for at least 2 years; State the obvious start by asking yourself: Case report form development represents a significant part of the clinical trial process and can affect study. You or your institution must be a fellow of bmj case reports in order to submit. Writing the core of your case report: Clinical findings and outcome step 1: Further information is available online on rates and how.
FREE 15+ Case Report Forms in PDF MS Word
Case Report Form Pdf Definitions case report form (crf) form on which individual participant data required by the study protocol are recorded. Writing the core of your case report: Case report form development represents a significant part of the clinical trial process and can affect study. Definitions case report form (crf) form on which individual participant data required by the study protocol are recorded. State the obvious start by asking yourself: Further information is available online on rates and how. Case report form (crf) is a specialized document in clinical research. 1.2 the case report form (crf) is a data collection tool used to capture the required data, as defined by the protocol, for each individual subject. Clinical findings and outcome step 1: Case report forms (crfs) are used to collect data in clinical research. You or your institution must be a fellow of bmj case reports in order to submit. It may be a paper. To be considered to be not of childbearing potential, the subject must be postmenopausal for at least 2 years;
From formspal.com
Case Report Form ≡ Fill Out Printable PDF Forms Online Case Report Form Pdf You or your institution must be a fellow of bmj case reports in order to submit. State the obvious start by asking yourself: Definitions case report form (crf) form on which individual participant data required by the study protocol are recorded. 1.2 the case report form (crf) is a data collection tool used to capture the required data, as defined. Case Report Form Pdf.
From www.scribd.com
Case Report Form PDF Dose (Biochemistry) Chemistry Case Report Form Pdf Writing the core of your case report: Case report form development represents a significant part of the clinical trial process and can affect study. You or your institution must be a fellow of bmj case reports in order to submit. Further information is available online on rates and how. 1.2 the case report form (crf) is a data collection tool. Case Report Form Pdf.
From www.swiftsparks.com
Case Report Form Template PROFESSIONAL TEMPLATES PROFESSIONAL TEMPLATES Case Report Form Pdf Case report form (crf) is a specialized document in clinical research. Further information is available online on rates and how. Case report form development represents a significant part of the clinical trial process and can affect study. Case report forms (crfs) are used to collect data in clinical research. You or your institution must be a fellow of bmj case. Case Report Form Pdf.
From www.researchgate.net
(PDF) Basics of case report form designing in clinical research Case Report Form Pdf Writing the core of your case report: State the obvious start by asking yourself: Case report form (crf) is a specialized document in clinical research. It may be a paper. You or your institution must be a fellow of bmj case reports in order to submit. To be considered to be not of childbearing potential, the subject must be postmenopausal. Case Report Form Pdf.
From www.pinterest.com
Free 15+ Case Report Forms In Pdf Ms Word with regard to Case Report Case Report Form Pdf 1.2 the case report form (crf) is a data collection tool used to capture the required data, as defined by the protocol, for each individual subject. It may be a paper. Definitions case report form (crf) form on which individual participant data required by the study protocol are recorded. Clinical findings and outcome step 1: You or your institution must. Case Report Form Pdf.
From www.sampletemplates.com
FREE 12+ Sample Case Report Templates in PDF MS Word Google Docs Case Report Form Pdf State the obvious start by asking yourself: Case report form development represents a significant part of the clinical trial process and can affect study. 1.2 the case report form (crf) is a data collection tool used to capture the required data, as defined by the protocol, for each individual subject. Definitions case report form (crf) form on which individual participant. Case Report Form Pdf.
From pray.gelorailmu.com
Free 15+ Case Report Forms In Pdf Ms Word inside Case Report Form Case Report Form Pdf Case report form (crf) is a specialized document in clinical research. You or your institution must be a fellow of bmj case reports in order to submit. Further information is available online on rates and how. It may be a paper. Definitions case report form (crf) form on which individual participant data required by the study protocol are recorded. Case. Case Report Form Pdf.
From www.sampleforms.com
FREE 15+ Case Report Forms in PDF MS Word Case Report Form Pdf Definitions case report form (crf) form on which individual participant data required by the study protocol are recorded. Further information is available online on rates and how. To be considered to be not of childbearing potential, the subject must be postmenopausal for at least 2 years; Clinical findings and outcome step 1: Case report forms (crfs) are used to collect. Case Report Form Pdf.
From www.sampletemplates.com
FREE 12+ Sample Case Report Templates in PDF MS Word Google Docs Case Report Form Pdf Clinical findings and outcome step 1: To be considered to be not of childbearing potential, the subject must be postmenopausal for at least 2 years; Case report forms (crfs) are used to collect data in clinical research. Case report form development represents a significant part of the clinical trial process and can affect study. It may be a paper. Case. Case Report Form Pdf.
From mundoplantillas.com
49 ejemplos y plantillas de estudios de casos gratuitos Mundo Plantillas Case Report Form Pdf State the obvious start by asking yourself: It may be a paper. 1.2 the case report form (crf) is a data collection tool used to capture the required data, as defined by the protocol, for each individual subject. Case report forms (crfs) are used to collect data in clinical research. Case report form development represents a significant part of the. Case Report Form Pdf.
From templatelab.com
49 Free Case Study Templates ( + Case Study Format Examples + ) Case Report Form Pdf Clinical findings and outcome step 1: State the obvious start by asking yourself: It may be a paper. Case report form (crf) is a specialized document in clinical research. Definitions case report form (crf) form on which individual participant data required by the study protocol are recorded. Case report forms (crfs) are used to collect data in clinical research. You. Case Report Form Pdf.
From www.jotform.com
Medical Case Report PDF Templates Jotform Case Report Form Pdf Definitions case report form (crf) form on which individual participant data required by the study protocol are recorded. You or your institution must be a fellow of bmj case reports in order to submit. State the obvious start by asking yourself: Further information is available online on rates and how. It may be a paper. Case report form development represents. Case Report Form Pdf.
From templatedbc.web.app
Crf Case Report Form Templates PrintableDB.web.app Case Report Form Pdf Writing the core of your case report: Clinical findings and outcome step 1: Further information is available online on rates and how. Case report form (crf) is a specialized document in clinical research. Definitions case report form (crf) form on which individual participant data required by the study protocol are recorded. Case report forms (crfs) are used to collect data. Case Report Form Pdf.
From www.sampletemplates.com
FREE 12+ Sample Case Report Templates in PDF MS Word Google Docs Case Report Form Pdf It may be a paper. Writing the core of your case report: 1.2 the case report form (crf) is a data collection tool used to capture the required data, as defined by the protocol, for each individual subject. Clinical findings and outcome step 1: Case report forms (crfs) are used to collect data in clinical research. Case report form (crf). Case Report Form Pdf.
From www.sampletemplates.com
FREE 12+ Sample Case Report Templates in PDF MS Word Google Docs Case Report Form Pdf Case report form development represents a significant part of the clinical trial process and can affect study. 1.2 the case report form (crf) is a data collection tool used to capture the required data, as defined by the protocol, for each individual subject. Case report forms (crfs) are used to collect data in clinical research. Clinical findings and outcome step. Case Report Form Pdf.
From www.sampleforms.com
FREE 15+ Case Report Forms in PDF MS Word Case Report Form Pdf Writing the core of your case report: Clinical findings and outcome step 1: Case report forms (crfs) are used to collect data in clinical research. Case report form (crf) is a specialized document in clinical research. State the obvious start by asking yourself: You or your institution must be a fellow of bmj case reports in order to submit. To. Case Report Form Pdf.
From www.sampleforms.com
What Is a Case Report Form? [ Importance, Tips, Samples ] Case Report Form Pdf Case report form (crf) is a specialized document in clinical research. Writing the core of your case report: You or your institution must be a fellow of bmj case reports in order to submit. Definitions case report form (crf) form on which individual participant data required by the study protocol are recorded. To be considered to be not of childbearing. Case Report Form Pdf.
From www.researchgate.net
(PDF) Case Report Patient Consent Form Blanc Case Report Form Pdf Definitions case report form (crf) form on which individual participant data required by the study protocol are recorded. Case report form (crf) is a specialized document in clinical research. Case report forms (crfs) are used to collect data in clinical research. Further information is available online on rates and how. It may be a paper. 1.2 the case report form. Case Report Form Pdf.
From www.sampleforms.com
FREE 15+ Case Report Forms in PDF MS Word Case Report Form Pdf Clinical findings and outcome step 1: To be considered to be not of childbearing potential, the subject must be postmenopausal for at least 2 years; State the obvious start by asking yourself: Case report forms (crfs) are used to collect data in clinical research. You or your institution must be a fellow of bmj case reports in order to submit.. Case Report Form Pdf.
From www.sampleforms.com
FREE 15+ Case Report Forms in PDF MS Word Case Report Form Pdf Definitions case report form (crf) form on which individual participant data required by the study protocol are recorded. Writing the core of your case report: Case report form development represents a significant part of the clinical trial process and can affect study. State the obvious start by asking yourself: To be considered to be not of childbearing potential, the subject. Case Report Form Pdf.
From www.sampletemplates.com
FREE 12+ Sample Case Report Templates in PDF MS Word Google Docs Case Report Form Pdf Case report form development represents a significant part of the clinical trial process and can affect study. Clinical findings and outcome step 1: Case report forms (crfs) are used to collect data in clinical research. You or your institution must be a fellow of bmj case reports in order to submit. It may be a paper. Definitions case report form. Case Report Form Pdf.
From www.pdffiller.com
Embalming Case Report Pdf Fill Online, Printable, Fillable, Blank Case Report Form Pdf Case report forms (crfs) are used to collect data in clinical research. 1.2 the case report form (crf) is a data collection tool used to capture the required data, as defined by the protocol, for each individual subject. To be considered to be not of childbearing potential, the subject must be postmenopausal for at least 2 years; Clinical findings and. Case Report Form Pdf.
From support.theboogaloo.org
Free 15+ Case Report Forms In Pdf Ms Word inside Case Report Form Case Report Form Pdf Case report form development represents a significant part of the clinical trial process and can affect study. State the obvious start by asking yourself: Clinical findings and outcome step 1: To be considered to be not of childbearing potential, the subject must be postmenopausal for at least 2 years; Definitions case report form (crf) form on which individual participant data. Case Report Form Pdf.
From pray.gelorailmu.com
Case Report Form Template Case Report Form Pdf Case report form (crf) is a specialized document in clinical research. Definitions case report form (crf) form on which individual participant data required by the study protocol are recorded. Case report form development represents a significant part of the clinical trial process and can affect study. Case report forms (crfs) are used to collect data in clinical research. 1.2 the. Case Report Form Pdf.
From www.sampleforms.com
FREE 15+ Case Report Forms in PDF MS Word Case Report Form Pdf Definitions case report form (crf) form on which individual participant data required by the study protocol are recorded. You or your institution must be a fellow of bmj case reports in order to submit. Clinical findings and outcome step 1: Further information is available online on rates and how. 1.2 the case report form (crf) is a data collection tool. Case Report Form Pdf.
From id.scribd.com
Case Report Form PDF Case Report Form Pdf It may be a paper. Case report form development represents a significant part of the clinical trial process and can affect study. State the obvious start by asking yourself: Further information is available online on rates and how. Writing the core of your case report: Case report form (crf) is a specialized document in clinical research. Case report forms (crfs). Case Report Form Pdf.
From id.scribd.com
Case Report Form PDF Case Report Form Pdf State the obvious start by asking yourself: 1.2 the case report form (crf) is a data collection tool used to capture the required data, as defined by the protocol, for each individual subject. Clinical findings and outcome step 1: Further information is available online on rates and how. You or your institution must be a fellow of bmj case reports. Case Report Form Pdf.
From formspal.com
Embalming Case Report Form ≡ Fill Out Printable PDF Forms Online Case Report Form Pdf Writing the core of your case report: State the obvious start by asking yourself: It may be a paper. Clinical findings and outcome step 1: Definitions case report form (crf) form on which individual participant data required by the study protocol are recorded. Case report form (crf) is a specialized document in clinical research. Further information is available online on. Case Report Form Pdf.
From www.sampleforms.com
FREE 15+ Case Report Forms in PDF MS Word Case Report Form Pdf State the obvious start by asking yourself: Clinical findings and outcome step 1: Case report form (crf) is a specialized document in clinical research. Case report form development represents a significant part of the clinical trial process and can affect study. Writing the core of your case report: 1.2 the case report form (crf) is a data collection tool used. Case Report Form Pdf.
From www.scribd.com
How To Write A Case Report For Publication PDF PDF Case Report Case Report Form Pdf Writing the core of your case report: 1.2 the case report form (crf) is a data collection tool used to capture the required data, as defined by the protocol, for each individual subject. Definitions case report form (crf) form on which individual participant data required by the study protocol are recorded. State the obvious start by asking yourself: You or. Case Report Form Pdf.
From www.researchgate.net
(PDF) Case Report Form Case Report Form Pdf State the obvious start by asking yourself: Case report form (crf) is a specialized document in clinical research. Clinical findings and outcome step 1: Case report forms (crfs) are used to collect data in clinical research. Writing the core of your case report: You or your institution must be a fellow of bmj case reports in order to submit. It. Case Report Form Pdf.
From www.sampletemplates.com
FREE 12+ Sample Case Report Templates in PDF MS Word Google Docs Case Report Form Pdf Writing the core of your case report: Definitions case report form (crf) form on which individual participant data required by the study protocol are recorded. You or your institution must be a fellow of bmj case reports in order to submit. Further information is available online on rates and how. Clinical findings and outcome step 1: Case report form development. Case Report Form Pdf.
From www.sampleforms.com
FREE 15+ Case Report Forms in PDF MS Word Case Report Form Pdf Clinical findings and outcome step 1: Case report forms (crfs) are used to collect data in clinical research. Writing the core of your case report: Case report form development represents a significant part of the clinical trial process and can affect study. State the obvious start by asking yourself: It may be a paper. Case report form (crf) is a. Case Report Form Pdf.
From www.signnow.com
Tickborne Disease Case Report Form PDF Health State Mn Fill Out and Case Report Form Pdf Writing the core of your case report: Clinical findings and outcome step 1: Case report form (crf) is a specialized document in clinical research. Case report form development represents a significant part of the clinical trial process and can affect study. Further information is available online on rates and how. To be considered to be not of childbearing potential, the. Case Report Form Pdf.
From www.sampleforms.com
FREE 15+ Case Report Forms in PDF MS Word Case Report Form Pdf State the obvious start by asking yourself: Case report forms (crfs) are used to collect data in clinical research. Case report form development represents a significant part of the clinical trial process and can affect study. Writing the core of your case report: Case report form (crf) is a specialized document in clinical research. Further information is available online on. Case Report Form Pdf.