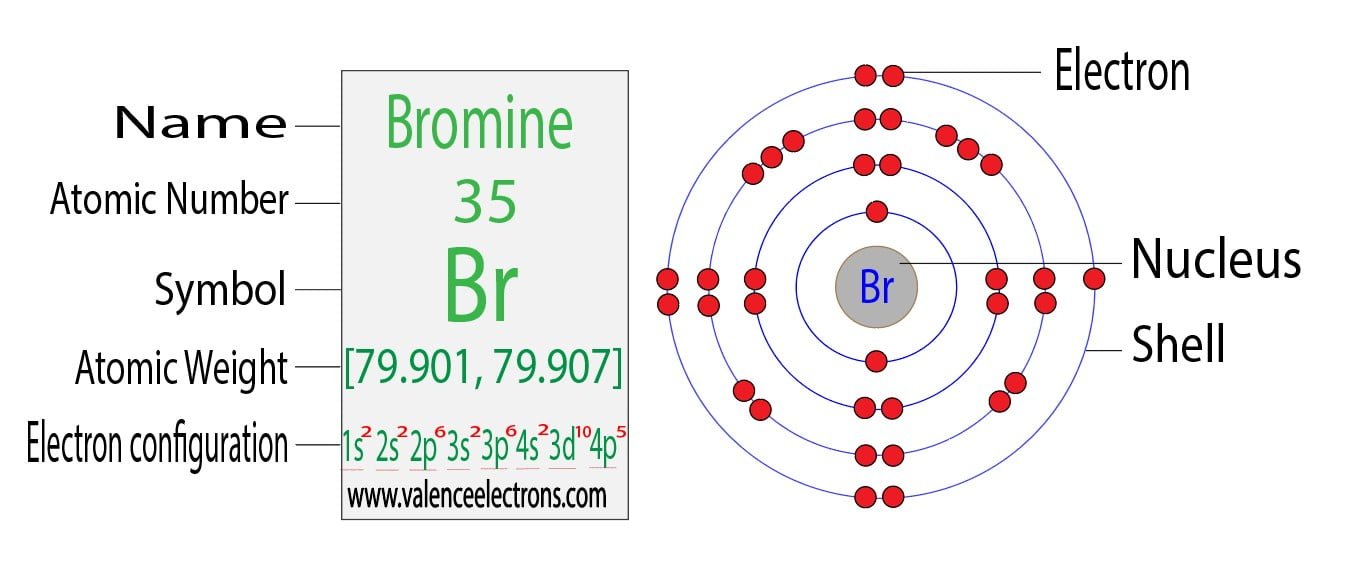
Full Electron Configuration For Bromine . Electron configuration of bromine (br) is [ar] 3d¹⁰ 4s² 4p⁵, indicating that br has 35 electrons distributed among its atomic orbitals. Bromine belongs to group 17 which is known as halogens. The bromine electron configuration, denoted as 4s2 3d10 4p5 or 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p5, showcases the precise placement of electrons within the atom. The electron configuration of bromine is #1s^2 2s^2 2p^6 3s^2 3p^6 4s^2 3d^10 4p^5#. Electronic configuration of group 17: Also, we will provide the pictures of the same. Please read the full article below for more information. All you need to do is work your way across the periodic table filling the orbitals as. The elements of group 17 have seven. The shorthand electron configuration (or noble gas configuration) as well as full electron configuration is also mentioned in the. What is the electron configuration for bromine? In this article today we are going to tell you about the electron configuration of bromine, its orbital diagram, and valence electron. This can be shortened to #[ar] 4s^2 3d^10 4p^5#.
from www.animalia-life.club
The shorthand electron configuration (or noble gas configuration) as well as full electron configuration is also mentioned in the. What is the electron configuration for bromine? Please read the full article below for more information. This can be shortened to #[ar] 4s^2 3d^10 4p^5#. Electronic configuration of group 17: Bromine belongs to group 17 which is known as halogens. In this article today we are going to tell you about the electron configuration of bromine, its orbital diagram, and valence electron. The elements of group 17 have seven. The bromine electron configuration, denoted as 4s2 3d10 4p5 or 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p5, showcases the precise placement of electrons within the atom. All you need to do is work your way across the periodic table filling the orbitals as.
Electron Configuration For Bromine
Full Electron Configuration For Bromine Please read the full article below for more information. All you need to do is work your way across the periodic table filling the orbitals as. Electronic configuration of group 17: The electron configuration of bromine is #1s^2 2s^2 2p^6 3s^2 3p^6 4s^2 3d^10 4p^5#. The elements of group 17 have seven. Electron configuration of bromine (br) is [ar] 3d¹⁰ 4s² 4p⁵, indicating that br has 35 electrons distributed among its atomic orbitals. What is the electron configuration for bromine? The bromine electron configuration, denoted as 4s2 3d10 4p5 or 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p5, showcases the precise placement of electrons within the atom. This can be shortened to #[ar] 4s^2 3d^10 4p^5#. In this article today we are going to tell you about the electron configuration of bromine, its orbital diagram, and valence electron. Please read the full article below for more information. Also, we will provide the pictures of the same. The shorthand electron configuration (or noble gas configuration) as well as full electron configuration is also mentioned in the. Bromine belongs to group 17 which is known as halogens.
From www.orangatame.com
How to Write Ground State Electron Configuration in Chemistry Full Electron Configuration For Bromine Electron configuration of bromine (br) is [ar] 3d¹⁰ 4s² 4p⁵, indicating that br has 35 electrons distributed among its atomic orbitals. Bromine belongs to group 17 which is known as halogens. Also, we will provide the pictures of the same. The electron configuration of bromine is #1s^2 2s^2 2p^6 3s^2 3p^6 4s^2 3d^10 4p^5#. What is the electron configuration for. Full Electron Configuration For Bromine.
From www.animalia-life.club
Electron Configuration For Bromine Full Electron Configuration For Bromine What is the electron configuration for bromine? The shorthand electron configuration (or noble gas configuration) as well as full electron configuration is also mentioned in the. Bromine belongs to group 17 which is known as halogens. All you need to do is work your way across the periodic table filling the orbitals as. This can be shortened to #[ar] 4s^2. Full Electron Configuration For Bromine.
From www.animalia-life.club
Electron Configuration For Bromine Full Electron Configuration For Bromine The elements of group 17 have seven. Bromine belongs to group 17 which is known as halogens. The shorthand electron configuration (or noble gas configuration) as well as full electron configuration is also mentioned in the. Electronic configuration of group 17: What is the electron configuration for bromine? Electron configuration of bromine (br) is [ar] 3d¹⁰ 4s² 4p⁵, indicating that. Full Electron Configuration For Bromine.
From ar.inspiredpencil.com
Electron Configuration For Bromine Full Electron Configuration For Bromine This can be shortened to #[ar] 4s^2 3d^10 4p^5#. The electron configuration of bromine is #1s^2 2s^2 2p^6 3s^2 3p^6 4s^2 3d^10 4p^5#. The shorthand electron configuration (or noble gas configuration) as well as full electron configuration is also mentioned in the. What is the electron configuration for bromine? Electron configuration of bromine (br) is [ar] 3d¹⁰ 4s² 4p⁵, indicating. Full Electron Configuration For Bromine.
From www.youtube.com
Electron Configuration of Bromine Br Lesson YouTube Full Electron Configuration For Bromine In this article today we are going to tell you about the electron configuration of bromine, its orbital diagram, and valence electron. This can be shortened to #[ar] 4s^2 3d^10 4p^5#. The shorthand electron configuration (or noble gas configuration) as well as full electron configuration is also mentioned in the. The electron configuration of bromine is #1s^2 2s^2 2p^6 3s^2. Full Electron Configuration For Bromine.
From www.numerade.com
SOLVEDWrite the full electron configuration (1 s^2 2 s^2, etc. ) for Full Electron Configuration For Bromine This can be shortened to #[ar] 4s^2 3d^10 4p^5#. The shorthand electron configuration (or noble gas configuration) as well as full electron configuration is also mentioned in the. Please read the full article below for more information. Electronic configuration of group 17: All you need to do is work your way across the periodic table filling the orbitals as. The. Full Electron Configuration For Bromine.
From general.chemistrysteps.com
Electron Configurations of Ions Chemistry Steps Full Electron Configuration For Bromine This can be shortened to #[ar] 4s^2 3d^10 4p^5#. The bromine electron configuration, denoted as 4s2 3d10 4p5 or 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p5, showcases the precise placement of electrons within the atom. Please read the full article below for more information. What is the electron configuration for bromine? Electronic configuration of group 17: The elements of. Full Electron Configuration For Bromine.
From www.youtube.com
Br Electron Configuration (Bromide Ion) YouTube Full Electron Configuration For Bromine In this article today we are going to tell you about the electron configuration of bromine, its orbital diagram, and valence electron. Please read the full article below for more information. What is the electron configuration for bromine? Bromine belongs to group 17 which is known as halogens. Electronic configuration of group 17: The electron configuration of bromine is #1s^2. Full Electron Configuration For Bromine.
From www.alamy.com
Bromine (Br). Diagram of the nuclear composition, electron Full Electron Configuration For Bromine The shorthand electron configuration (or noble gas configuration) as well as full electron configuration is also mentioned in the. Electronic configuration of group 17: In this article today we are going to tell you about the electron configuration of bromine, its orbital diagram, and valence electron. This can be shortened to #[ar] 4s^2 3d^10 4p^5#. All you need to do. Full Electron Configuration For Bromine.
From www.animalia-life.club
Electron Configuration For Bromine Full Electron Configuration For Bromine Also, we will provide the pictures of the same. The shorthand electron configuration (or noble gas configuration) as well as full electron configuration is also mentioned in the. This can be shortened to #[ar] 4s^2 3d^10 4p^5#. The bromine electron configuration, denoted as 4s2 3d10 4p5 or 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p5, showcases the precise placement of. Full Electron Configuration For Bromine.
From www.alamy.com
Bromine (Br). Diagram of the nuclear composition and electron Full Electron Configuration For Bromine What is the electron configuration for bromine? The bromine electron configuration, denoted as 4s2 3d10 4p5 or 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p5, showcases the precise placement of electrons within the atom. The shorthand electron configuration (or noble gas configuration) as well as full electron configuration is also mentioned in the. Electronic configuration of group 17: In this. Full Electron Configuration For Bromine.
From www.animalia-life.club
Electron Configuration For Bromine Full Electron Configuration For Bromine Bromine belongs to group 17 which is known as halogens. The elements of group 17 have seven. The bromine electron configuration, denoted as 4s2 3d10 4p5 or 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p5, showcases the precise placement of electrons within the atom. The electron configuration of bromine is #1s^2 2s^2 2p^6 3s^2 3p^6 4s^2 3d^10 4p^5#. This can. Full Electron Configuration For Bromine.
From www.thoughtco.com
Atom Diagrams Electron Configurations of the Elements Full Electron Configuration For Bromine Electronic configuration of group 17: Please read the full article below for more information. This can be shortened to #[ar] 4s^2 3d^10 4p^5#. Bromine belongs to group 17 which is known as halogens. In this article today we are going to tell you about the electron configuration of bromine, its orbital diagram, and valence electron. The shorthand electron configuration (or. Full Electron Configuration For Bromine.
From www.animalia-life.club
Electron Configuration For Bromine Full Electron Configuration For Bromine Electron configuration of bromine (br) is [ar] 3d¹⁰ 4s² 4p⁵, indicating that br has 35 electrons distributed among its atomic orbitals. In this article today we are going to tell you about the electron configuration of bromine, its orbital diagram, and valence electron. The shorthand electron configuration (or noble gas configuration) as well as full electron configuration is also mentioned. Full Electron Configuration For Bromine.
From www.animalia-life.club
Electron Configuration For Bromine Full Electron Configuration For Bromine The elements of group 17 have seven. What is the electron configuration for bromine? In this article today we are going to tell you about the electron configuration of bromine, its orbital diagram, and valence electron. The bromine electron configuration, denoted as 4s2 3d10 4p5 or 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p5, showcases the precise placement of electrons. Full Electron Configuration For Bromine.
From www.schoolmykids.com
Compare Bromine vs Iodine Periodic Table Element Comparison Compare Full Electron Configuration For Bromine This can be shortened to #[ar] 4s^2 3d^10 4p^5#. The electron configuration of bromine is #1s^2 2s^2 2p^6 3s^2 3p^6 4s^2 3d^10 4p^5#. Bromine belongs to group 17 which is known as halogens. All you need to do is work your way across the periodic table filling the orbitals as. In this article today we are going to tell you. Full Electron Configuration For Bromine.
From www.animalia-life.club
Electron Configuration For Bromine Full Electron Configuration For Bromine The elements of group 17 have seven. What is the electron configuration for bromine? All you need to do is work your way across the periodic table filling the orbitals as. Also, we will provide the pictures of the same. Please read the full article below for more information. This can be shortened to #[ar] 4s^2 3d^10 4p^5#. Electronic configuration. Full Electron Configuration For Bromine.
From ar.inspiredpencil.com
Atomic Structure Of Bromine Full Electron Configuration For Bromine All you need to do is work your way across the periodic table filling the orbitals as. Electron configuration of bromine (br) is [ar] 3d¹⁰ 4s² 4p⁵, indicating that br has 35 electrons distributed among its atomic orbitals. What is the electron configuration for bromine? The elements of group 17 have seven. Also, we will provide the pictures of the. Full Electron Configuration For Bromine.
From www.animalia-life.club
Electron Configuration For Bromine Full Electron Configuration For Bromine Electronic configuration of group 17: Also, we will provide the pictures of the same. What is the electron configuration for bromine? All you need to do is work your way across the periodic table filling the orbitals as. Electron configuration of bromine (br) is [ar] 3d¹⁰ 4s² 4p⁵, indicating that br has 35 electrons distributed among its atomic orbitals. The. Full Electron Configuration For Bromine.
From carisca.github.io
Cr2+ Ground State Electron Configuration Electron Configurations Full Electron Configuration For Bromine The electron configuration of bromine is #1s^2 2s^2 2p^6 3s^2 3p^6 4s^2 3d^10 4p^5#. In this article today we are going to tell you about the electron configuration of bromine, its orbital diagram, and valence electron. All you need to do is work your way across the periodic table filling the orbitals as. The bromine electron configuration, denoted as 4s2. Full Electron Configuration For Bromine.
From www.webelements.com
Elements Periodic Table » Bromine » properties of free atoms Full Electron Configuration For Bromine The electron configuration of bromine is #1s^2 2s^2 2p^6 3s^2 3p^6 4s^2 3d^10 4p^5#. The shorthand electron configuration (or noble gas configuration) as well as full electron configuration is also mentioned in the. Also, we will provide the pictures of the same. Electron configuration of bromine (br) is [ar] 3d¹⁰ 4s² 4p⁵, indicating that br has 35 electrons distributed among. Full Electron Configuration For Bromine.
From ar.inspiredpencil.com
Electron Configuration For Bromine Full Electron Configuration For Bromine What is the electron configuration for bromine? In this article today we are going to tell you about the electron configuration of bromine, its orbital diagram, and valence electron. Also, we will provide the pictures of the same. Electronic configuration of group 17: The electron configuration of bromine is #1s^2 2s^2 2p^6 3s^2 3p^6 4s^2 3d^10 4p^5#. Please read the. Full Electron Configuration For Bromine.
From periodictable.me
Bromine Electron Configuration (Br) with Orbital Diagram Full Electron Configuration For Bromine In this article today we are going to tell you about the electron configuration of bromine, its orbital diagram, and valence electron. Electron configuration of bromine (br) is [ar] 3d¹⁰ 4s² 4p⁵, indicating that br has 35 electrons distributed among its atomic orbitals. All you need to do is work your way across the periodic table filling the orbitals as.. Full Electron Configuration For Bromine.
From ar.inspiredpencil.com
Electron Configuration For Bromine Full Electron Configuration For Bromine The shorthand electron configuration (or noble gas configuration) as well as full electron configuration is also mentioned in the. Electron configuration of bromine (br) is [ar] 3d¹⁰ 4s² 4p⁵, indicating that br has 35 electrons distributed among its atomic orbitals. Bromine belongs to group 17 which is known as halogens. The bromine electron configuration, denoted as 4s2 3d10 4p5 or. Full Electron Configuration For Bromine.
From ar.inspiredpencil.com
Electron Configuration Of Bromine Full Electron Configuration For Bromine The bromine electron configuration, denoted as 4s2 3d10 4p5 or 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p5, showcases the precise placement of electrons within the atom. The elements of group 17 have seven. Also, we will provide the pictures of the same. All you need to do is work your way across the periodic table filling the orbitals as.. Full Electron Configuration For Bromine.
From www.animalia-life.club
Electron Configuration For Bromine Full Electron Configuration For Bromine The electron configuration of bromine is #1s^2 2s^2 2p^6 3s^2 3p^6 4s^2 3d^10 4p^5#. Please read the full article below for more information. This can be shortened to #[ar] 4s^2 3d^10 4p^5#. Also, we will provide the pictures of the same. Electronic configuration of group 17: All you need to do is work your way across the periodic table filling. Full Electron Configuration For Bromine.
From valenceelectrons.com
Complete Electron Configuration for Bromine (Br, Br ion) Full Electron Configuration For Bromine What is the electron configuration for bromine? All you need to do is work your way across the periodic table filling the orbitals as. The shorthand electron configuration (or noble gas configuration) as well as full electron configuration is also mentioned in the. Bromine belongs to group 17 which is known as halogens. This can be shortened to #[ar] 4s^2. Full Electron Configuration For Bromine.
From socratic.org
Why does fluorine have a higher ionization energy than bromine? Socratic Full Electron Configuration For Bromine Please read the full article below for more information. Also, we will provide the pictures of the same. This can be shortened to #[ar] 4s^2 3d^10 4p^5#. Electron configuration of bromine (br) is [ar] 3d¹⁰ 4s² 4p⁵, indicating that br has 35 electrons distributed among its atomic orbitals. The elements of group 17 have seven. The electron configuration of bromine. Full Electron Configuration For Bromine.
From www.animalia-life.club
Electron Configuration For Bromine Full Electron Configuration For Bromine The electron configuration of bromine is #1s^2 2s^2 2p^6 3s^2 3p^6 4s^2 3d^10 4p^5#. All you need to do is work your way across the periodic table filling the orbitals as. Electronic configuration of group 17: The shorthand electron configuration (or noble gas configuration) as well as full electron configuration is also mentioned in the. In this article today we. Full Electron Configuration For Bromine.
From periodictable.me
Bromine Electron Configuration (Br) with Orbital Diagram Full Electron Configuration For Bromine In this article today we are going to tell you about the electron configuration of bromine, its orbital diagram, and valence electron. The bromine electron configuration, denoted as 4s2 3d10 4p5 or 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p5, showcases the precise placement of electrons within the atom. Electronic configuration of group 17: Please read the full article below. Full Electron Configuration For Bromine.
From www.pinterest.com
Bromine Facts Atomic Number 35 and Element Symbol Br Full Electron Configuration For Bromine Also, we will provide the pictures of the same. Electronic configuration of group 17: The shorthand electron configuration (or noble gas configuration) as well as full electron configuration is also mentioned in the. The electron configuration of bromine is #1s^2 2s^2 2p^6 3s^2 3p^6 4s^2 3d^10 4p^5#. The bromine electron configuration, denoted as 4s2 3d10 4p5 or 1s2 2s2 2p6. Full Electron Configuration For Bromine.
From www.nuclear-power.com
Bromine Electron Affinity Electronegativity Ionization Energy of Full Electron Configuration For Bromine All you need to do is work your way across the periodic table filling the orbitals as. The electron configuration of bromine is #1s^2 2s^2 2p^6 3s^2 3p^6 4s^2 3d^10 4p^5#. Bromine belongs to group 17 which is known as halogens. Electron configuration of bromine (br) is [ar] 3d¹⁰ 4s² 4p⁵, indicating that br has 35 electrons distributed among its. Full Electron Configuration For Bromine.
From www.youtube.com
Full and Abbreviated Electron Configuration of Bromine Br YouTube Full Electron Configuration For Bromine Electron configuration of bromine (br) is [ar] 3d¹⁰ 4s² 4p⁵, indicating that br has 35 electrons distributed among its atomic orbitals. Electronic configuration of group 17: What is the electron configuration for bromine? Please read the full article below for more information. Bromine belongs to group 17 which is known as halogens. Also, we will provide the pictures of the. Full Electron Configuration For Bromine.
From periodictable.me
How Do We Find The Electron Configuration For Bromine Dynamic Full Electron Configuration For Bromine The shorthand electron configuration (or noble gas configuration) as well as full electron configuration is also mentioned in the. Also, we will provide the pictures of the same. Electronic configuration of group 17: What is the electron configuration for bromine? The elements of group 17 have seven. Electron configuration of bromine (br) is [ar] 3d¹⁰ 4s² 4p⁵, indicating that br. Full Electron Configuration For Bromine.
From schematron.org
Bromine Orbital Diagram Wiring Diagram Pictures Full Electron Configuration For Bromine All you need to do is work your way across the periodic table filling the orbitals as. Bromine belongs to group 17 which is known as halogens. Electronic configuration of group 17: The shorthand electron configuration (or noble gas configuration) as well as full electron configuration is also mentioned in the. Electron configuration of bromine (br) is [ar] 3d¹⁰ 4s². Full Electron Configuration For Bromine.