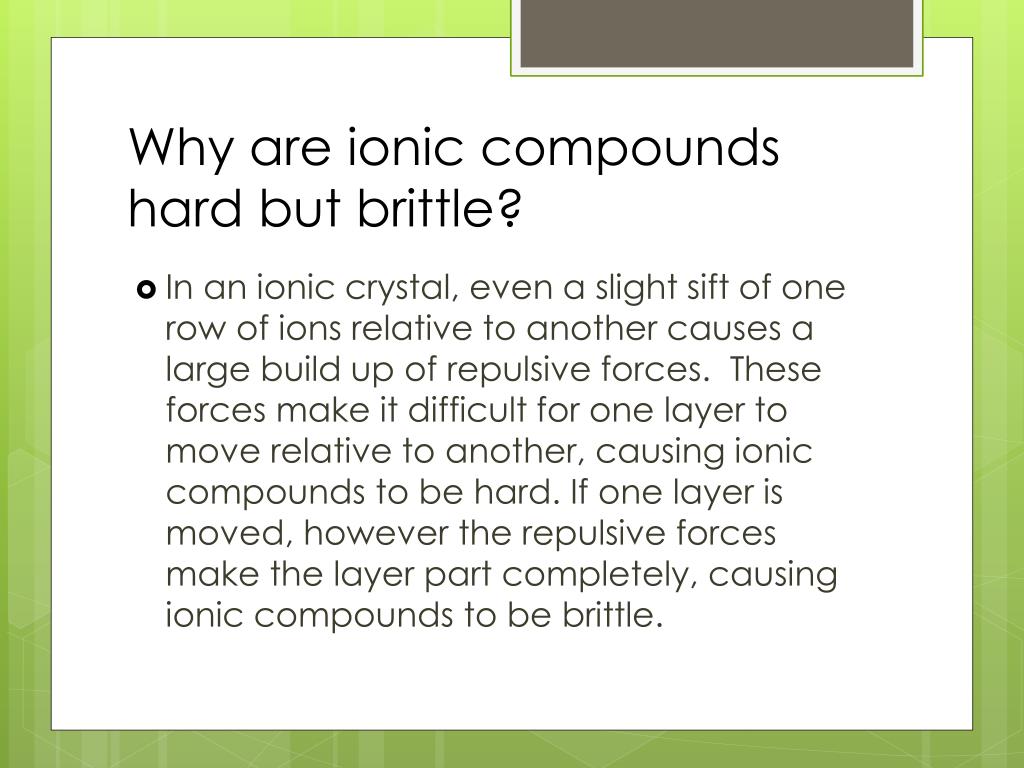
Are Ionic Compounds Brittle Or Malleable . Ionic compounds form crystals, typically have high melting and boiling points, are usually hard and brittle, and form electrolytes in water. It takes a large amount of mechanical force, such as striking a crystal with a hammer, to force one layer of ions to. Ionic compounds are generally hard, but brittle. When an external force is applied, it can shift the layers. Covalent compounds usually have lower melting and boiling points than ionic compounds, are softer, and are electrical insulators. The reasons why things are brittle have more to do with the bulk structure of the material and less to do with the chemical. Ionic compounds have high melting and boiling points, so they are in the solid state at room temperature. Most covalent compounds consist of nonmetals bonded to one another. No, ionic compounds are not malleable. They are brittle and can shatter when struck or subjected to stress. Ionic compounds dissociate into ions when dissolved in water. Ionic compounds are hard and brittle. Ionic compounds are brittle because they have a crystal lattice structure.
from www.slideserve.com
Covalent compounds usually have lower melting and boiling points than ionic compounds, are softer, and are electrical insulators. Most covalent compounds consist of nonmetals bonded to one another. Ionic compounds are generally hard, but brittle. It takes a large amount of mechanical force, such as striking a crystal with a hammer, to force one layer of ions to. When an external force is applied, it can shift the layers. No, ionic compounds are not malleable. Ionic compounds have high melting and boiling points, so they are in the solid state at room temperature. Ionic compounds form crystals, typically have high melting and boiling points, are usually hard and brittle, and form electrolytes in water. Ionic compounds dissociate into ions when dissolved in water. Ionic compounds are hard and brittle.
PPT Ionic and Covalent bonds PowerPoint Presentation, free download
Are Ionic Compounds Brittle Or Malleable Ionic compounds have high melting and boiling points, so they are in the solid state at room temperature. Covalent compounds usually have lower melting and boiling points than ionic compounds, are softer, and are electrical insulators. The reasons why things are brittle have more to do with the bulk structure of the material and less to do with the chemical. Ionic compounds have high melting and boiling points, so they are in the solid state at room temperature. It takes a large amount of mechanical force, such as striking a crystal with a hammer, to force one layer of ions to. When an external force is applied, it can shift the layers. Ionic compounds form crystals, typically have high melting and boiling points, are usually hard and brittle, and form electrolytes in water. Ionic compounds are generally hard, but brittle. No, ionic compounds are not malleable. Ionic compounds are brittle because they have a crystal lattice structure. Ionic compounds are hard and brittle. Ionic compounds dissociate into ions when dissolved in water. They are brittle and can shatter when struck or subjected to stress. Most covalent compounds consist of nonmetals bonded to one another.
From slidetodoc.com
TYPES OF CHEMICAL BONDS IONIC BONDS COVALENT BONDS Are Ionic Compounds Brittle Or Malleable They are brittle and can shatter when struck or subjected to stress. It takes a large amount of mechanical force, such as striking a crystal with a hammer, to force one layer of ions to. Covalent compounds usually have lower melting and boiling points than ionic compounds, are softer, and are electrical insulators. When an external force is applied, it. Are Ionic Compounds Brittle Or Malleable.
From israelyouthsims.blogspot.com
Best Describes the Properties of Ionic Compounds Are Ionic Compounds Brittle Or Malleable Ionic compounds form crystals, typically have high melting and boiling points, are usually hard and brittle, and form electrolytes in water. Ionic compounds are brittle because they have a crystal lattice structure. Ionic compounds are generally hard, but brittle. No, ionic compounds are not malleable. It takes a large amount of mechanical force, such as striking a crystal with a. Are Ionic Compounds Brittle Or Malleable.
From www.hotzxgirl.com
What Are Ionic Compounds Definition Structure Properties Hot Sex Picture Are Ionic Compounds Brittle Or Malleable Ionic compounds form crystals, typically have high melting and boiling points, are usually hard and brittle, and form electrolytes in water. They are brittle and can shatter when struck or subjected to stress. Ionic compounds are hard and brittle. No, ionic compounds are not malleable. It takes a large amount of mechanical force, such as striking a crystal with a. Are Ionic Compounds Brittle Or Malleable.
From slideplayer.com
Chemistry Ionic Compounds. ppt download Are Ionic Compounds Brittle Or Malleable Ionic compounds dissociate into ions when dissolved in water. No, ionic compounds are not malleable. Most covalent compounds consist of nonmetals bonded to one another. Covalent compounds usually have lower melting and boiling points than ionic compounds, are softer, and are electrical insulators. They are brittle and can shatter when struck or subjected to stress. Ionic compounds are brittle because. Are Ionic Compounds Brittle Or Malleable.
From slideplayer.com
Ionic compounds names and formulas ppt download Are Ionic Compounds Brittle Or Malleable They are brittle and can shatter when struck or subjected to stress. The reasons why things are brittle have more to do with the bulk structure of the material and less to do with the chemical. Ionic compounds are brittle because they have a crystal lattice structure. Ionic compounds are hard and brittle. No, ionic compounds are not malleable. It. Are Ionic Compounds Brittle Or Malleable.
From slideplayer.com
Lewis Dot Structures And… Ionic & Metallic Bonding. ppt download Are Ionic Compounds Brittle Or Malleable They are brittle and can shatter when struck or subjected to stress. It takes a large amount of mechanical force, such as striking a crystal with a hammer, to force one layer of ions to. Ionic compounds dissociate into ions when dissolved in water. Ionic compounds are generally hard, but brittle. Ionic compounds have high melting and boiling points, so. Are Ionic Compounds Brittle Or Malleable.
From slideplayer.com
Comparison of Properties Ionic Compounds Covalent Compounds Metals Are Ionic Compounds Brittle Or Malleable Ionic compounds are brittle because they have a crystal lattice structure. The reasons why things are brittle have more to do with the bulk structure of the material and less to do with the chemical. Ionic compounds form crystals, typically have high melting and boiling points, are usually hard and brittle, and form electrolytes in water. Ionic compounds are generally. Are Ionic Compounds Brittle Or Malleable.
From www.slideserve.com
PPT Properties of ionic compounds PowerPoint Presentation ID1130121 Are Ionic Compounds Brittle Or Malleable They are brittle and can shatter when struck or subjected to stress. Ionic compounds form crystals, typically have high melting and boiling points, are usually hard and brittle, and form electrolytes in water. Ionic compounds are hard and brittle. The reasons why things are brittle have more to do with the bulk structure of the material and less to do. Are Ionic Compounds Brittle Or Malleable.
From www.youtube.com
Why ionic solids are brittle? YouTube Are Ionic Compounds Brittle Or Malleable No, ionic compounds are not malleable. Ionic compounds are generally hard, but brittle. Ionic compounds are hard and brittle. Ionic compounds have high melting and boiling points, so they are in the solid state at room temperature. Ionic compounds form crystals, typically have high melting and boiling points, are usually hard and brittle, and form electrolytes in water. Ionic compounds. Are Ionic Compounds Brittle Or Malleable.
From www.slideserve.com
PPT Chapter 15 Ionic Bonding and Ionic Compounds PowerPoint Are Ionic Compounds Brittle Or Malleable When an external force is applied, it can shift the layers. Ionic compounds have high melting and boiling points, so they are in the solid state at room temperature. They are brittle and can shatter when struck or subjected to stress. The reasons why things are brittle have more to do with the bulk structure of the material and less. Are Ionic Compounds Brittle Or Malleable.
From ibstudy.net
4.1 Ionic bonding and structure Wang's website Are Ionic Compounds Brittle Or Malleable No, ionic compounds are not malleable. They are brittle and can shatter when struck or subjected to stress. When an external force is applied, it can shift the layers. Covalent compounds usually have lower melting and boiling points than ionic compounds, are softer, and are electrical insulators. Ionic compounds form crystals, typically have high melting and boiling points, are usually. Are Ionic Compounds Brittle Or Malleable.
From www.slideserve.com
PPT Chapter 15 Ionic Bonding and Ionic Compounds PowerPoint Are Ionic Compounds Brittle Or Malleable Ionic compounds are generally hard, but brittle. Most covalent compounds consist of nonmetals bonded to one another. They are brittle and can shatter when struck or subjected to stress. Ionic compounds are hard and brittle. Ionic compounds have high melting and boiling points, so they are in the solid state at room temperature. It takes a large amount of mechanical. Are Ionic Compounds Brittle Or Malleable.
From www.slideserve.com
PPT Ionic and Covalent bonds PowerPoint Presentation, free download Are Ionic Compounds Brittle Or Malleable The reasons why things are brittle have more to do with the bulk structure of the material and less to do with the chemical. Ionic compounds are generally hard, but brittle. No, ionic compounds are not malleable. When an external force is applied, it can shift the layers. Ionic compounds dissociate into ions when dissolved in water. Ionic compounds form. Are Ionic Compounds Brittle Or Malleable.
From www.slideshare.net
Chemistry Chp 7 Ionic And Metallic Bonding PowerPoint Are Ionic Compounds Brittle Or Malleable No, ionic compounds are not malleable. Ionic compounds have high melting and boiling points, so they are in the solid state at room temperature. Ionic compounds are hard and brittle. The reasons why things are brittle have more to do with the bulk structure of the material and less to do with the chemical. Ionic compounds are brittle because they. Are Ionic Compounds Brittle Or Malleable.
From www.vrogue.co
What Are Ionic Compounds Definition Examples Reaction vrogue.co Are Ionic Compounds Brittle Or Malleable The reasons why things are brittle have more to do with the bulk structure of the material and less to do with the chemical. Covalent compounds usually have lower melting and boiling points than ionic compounds, are softer, and are electrical insulators. Most covalent compounds consist of nonmetals bonded to one another. Ionic compounds are brittle because they have a. Are Ionic Compounds Brittle Or Malleable.
From brainly.com
The table below shows selected properties of compounds that Are Ionic Compounds Brittle Or Malleable They are brittle and can shatter when struck or subjected to stress. Ionic compounds form crystals, typically have high melting and boiling points, are usually hard and brittle, and form electrolytes in water. The reasons why things are brittle have more to do with the bulk structure of the material and less to do with the chemical. Ionic compounds are. Are Ionic Compounds Brittle Or Malleable.
From www.slideserve.com
PPT Worksheet Chapter 12 Homework Key PowerPoint Presentation, free Are Ionic Compounds Brittle Or Malleable Most covalent compounds consist of nonmetals bonded to one another. It takes a large amount of mechanical force, such as striking a crystal with a hammer, to force one layer of ions to. No, ionic compounds are not malleable. Ionic compounds are generally hard, but brittle. They are brittle and can shatter when struck or subjected to stress. Covalent compounds. Are Ionic Compounds Brittle Or Malleable.
From chem.libretexts.org
3.1 Types of Chemical Compounds and their Formulas Chemistry LibreTexts Are Ionic Compounds Brittle Or Malleable When an external force is applied, it can shift the layers. Ionic compounds form crystals, typically have high melting and boiling points, are usually hard and brittle, and form electrolytes in water. Ionic compounds dissociate into ions when dissolved in water. Ionic compounds are generally hard, but brittle. Most covalent compounds consist of nonmetals bonded to one another. Ionic compounds. Are Ionic Compounds Brittle Or Malleable.
From www.slideserve.com
PPT Chapter 15 Ionic Bonding and Ionic Compounds PowerPoint Are Ionic Compounds Brittle Or Malleable Ionic compounds are brittle because they have a crystal lattice structure. Most covalent compounds consist of nonmetals bonded to one another. They are brittle and can shatter when struck or subjected to stress. Covalent compounds usually have lower melting and boiling points than ionic compounds, are softer, and are electrical insulators. Ionic compounds form crystals, typically have high melting and. Are Ionic Compounds Brittle Or Malleable.
From www.chegg.com
Solved Select all of the properties of ionic compounds. Are Ionic Compounds Brittle Or Malleable Ionic compounds are generally hard, but brittle. Ionic compounds are hard and brittle. Most covalent compounds consist of nonmetals bonded to one another. Ionic compounds dissociate into ions when dissolved in water. Ionic compounds have high melting and boiling points, so they are in the solid state at room temperature. Covalent compounds usually have lower melting and boiling points than. Are Ionic Compounds Brittle Or Malleable.
From chem.libretexts.org
Ionic Solids Chemistry LibreTexts Are Ionic Compounds Brittle Or Malleable Ionic compounds are hard and brittle. Ionic compounds dissociate into ions when dissolved in water. The reasons why things are brittle have more to do with the bulk structure of the material and less to do with the chemical. No, ionic compounds are not malleable. Ionic compounds form crystals, typically have high melting and boiling points, are usually hard and. Are Ionic Compounds Brittle Or Malleable.
From slideplayer.com
Ionic vs Molecular Compounds ppt download Are Ionic Compounds Brittle Or Malleable Ionic compounds are generally hard, but brittle. No, ionic compounds are not malleable. Ionic compounds form crystals, typically have high melting and boiling points, are usually hard and brittle, and form electrolytes in water. Ionic compounds are brittle because they have a crystal lattice structure. Ionic compounds have high melting and boiling points, so they are in the solid state. Are Ionic Compounds Brittle Or Malleable.
From www.slideserve.com
PPT Chapter 15 Ionic Bonding and Ionic Compounds PowerPoint Are Ionic Compounds Brittle Or Malleable Ionic compounds are brittle because they have a crystal lattice structure. No, ionic compounds are not malleable. When an external force is applied, it can shift the layers. The reasons why things are brittle have more to do with the bulk structure of the material and less to do with the chemical. Ionic compounds form crystals, typically have high melting. Are Ionic Compounds Brittle Or Malleable.
From www.chegg.com
Solved Which of the following compounds are ionic? Check all Are Ionic Compounds Brittle Or Malleable Ionic compounds dissociate into ions when dissolved in water. They are brittle and can shatter when struck or subjected to stress. When an external force is applied, it can shift the layers. Ionic compounds have high melting and boiling points, so they are in the solid state at room temperature. It takes a large amount of mechanical force, such as. Are Ionic Compounds Brittle Or Malleable.
From slideplayer.com
Chemical Bonding and Molecular Geometry ppt download Are Ionic Compounds Brittle Or Malleable Ionic compounds dissociate into ions when dissolved in water. Ionic compounds are generally hard, but brittle. The reasons why things are brittle have more to do with the bulk structure of the material and less to do with the chemical. Most covalent compounds consist of nonmetals bonded to one another. Ionic compounds form crystals, typically have high melting and boiling. Are Ionic Compounds Brittle Or Malleable.
From www.slideserve.com
PPT What are bonds? PowerPoint Presentation, free download ID5861644 Are Ionic Compounds Brittle Or Malleable Covalent compounds usually have lower melting and boiling points than ionic compounds, are softer, and are electrical insulators. Ionic compounds have high melting and boiling points, so they are in the solid state at room temperature. No, ionic compounds are not malleable. Ionic compounds form crystals, typically have high melting and boiling points, are usually hard and brittle, and form. Are Ionic Compounds Brittle Or Malleable.
From slidetodoc.com
Ionic Compounds Chapter 5 Ionic Compounds Ionic compounds Are Ionic Compounds Brittle Or Malleable Most covalent compounds consist of nonmetals bonded to one another. Ionic compounds are hard and brittle. They are brittle and can shatter when struck or subjected to stress. Ionic compounds are generally hard, but brittle. Ionic compounds are brittle because they have a crystal lattice structure. No, ionic compounds are not malleable. Ionic compounds have high melting and boiling points,. Are Ionic Compounds Brittle Or Malleable.
From www.slideserve.com
PPT Chapter 15 Ionic Bonding and Ionic Compounds PowerPoint Are Ionic Compounds Brittle Or Malleable They are brittle and can shatter when struck or subjected to stress. Most covalent compounds consist of nonmetals bonded to one another. It takes a large amount of mechanical force, such as striking a crystal with a hammer, to force one layer of ions to. No, ionic compounds are not malleable. Ionic compounds dissociate into ions when dissolved in water.. Are Ionic Compounds Brittle Or Malleable.
From slideplayer.com
Ionic, Covalent, Metallic ppt download Are Ionic Compounds Brittle Or Malleable Ionic compounds dissociate into ions when dissolved in water. No, ionic compounds are not malleable. They are brittle and can shatter when struck or subjected to stress. It takes a large amount of mechanical force, such as striking a crystal with a hammer, to force one layer of ions to. Ionic compounds are brittle because they have a crystal lattice. Are Ionic Compounds Brittle Or Malleable.
From slideplayer.com
Ionic Bonding/Naming. ppt download Are Ionic Compounds Brittle Or Malleable Ionic compounds are brittle because they have a crystal lattice structure. No, ionic compounds are not malleable. The reasons why things are brittle have more to do with the bulk structure of the material and less to do with the chemical. It takes a large amount of mechanical force, such as striking a crystal with a hammer, to force one. Are Ionic Compounds Brittle Or Malleable.
From brainly.in
Why ionic crystals are brittle in nature? Brainly.in Are Ionic Compounds Brittle Or Malleable Ionic compounds form crystals, typically have high melting and boiling points, are usually hard and brittle, and form electrolytes in water. The reasons why things are brittle have more to do with the bulk structure of the material and less to do with the chemical. Most covalent compounds consist of nonmetals bonded to one another. Covalent compounds usually have lower. Are Ionic Compounds Brittle Or Malleable.
From slideplayer.com
Ionic Bonding. ppt download Are Ionic Compounds Brittle Or Malleable Ionic compounds have high melting and boiling points, so they are in the solid state at room temperature. Ionic compounds dissociate into ions when dissolved in water. Ionic compounds are generally hard, but brittle. Ionic compounds are hard and brittle. It takes a large amount of mechanical force, such as striking a crystal with a hammer, to force one layer. Are Ionic Compounds Brittle Or Malleable.
From www.vrogue.co
What Are Ionic Compounds Definition Examples Reaction vrogue.co Are Ionic Compounds Brittle Or Malleable Covalent compounds usually have lower melting and boiling points than ionic compounds, are softer, and are electrical insulators. Ionic compounds dissociate into ions when dissolved in water. Ionic compounds are generally hard, but brittle. Ionic compounds are hard and brittle. It takes a large amount of mechanical force, such as striking a crystal with a hammer, to force one layer. Are Ionic Compounds Brittle Or Malleable.
From slideplayer.com
Notes Properties of Ionic Compounds 3 ppt download Are Ionic Compounds Brittle Or Malleable Ionic compounds dissociate into ions when dissolved in water. When an external force is applied, it can shift the layers. The reasons why things are brittle have more to do with the bulk structure of the material and less to do with the chemical. Most covalent compounds consist of nonmetals bonded to one another. Ionic compounds are hard and brittle.. Are Ionic Compounds Brittle Or Malleable.
From slideplayer.com
Ionic and Metallic Bonds ppt download Are Ionic Compounds Brittle Or Malleable Ionic compounds are generally hard, but brittle. When an external force is applied, it can shift the layers. It takes a large amount of mechanical force, such as striking a crystal with a hammer, to force one layer of ions to. No, ionic compounds are not malleable. Ionic compounds are hard and brittle. Ionic compounds dissociate into ions when dissolved. Are Ionic Compounds Brittle Or Malleable.