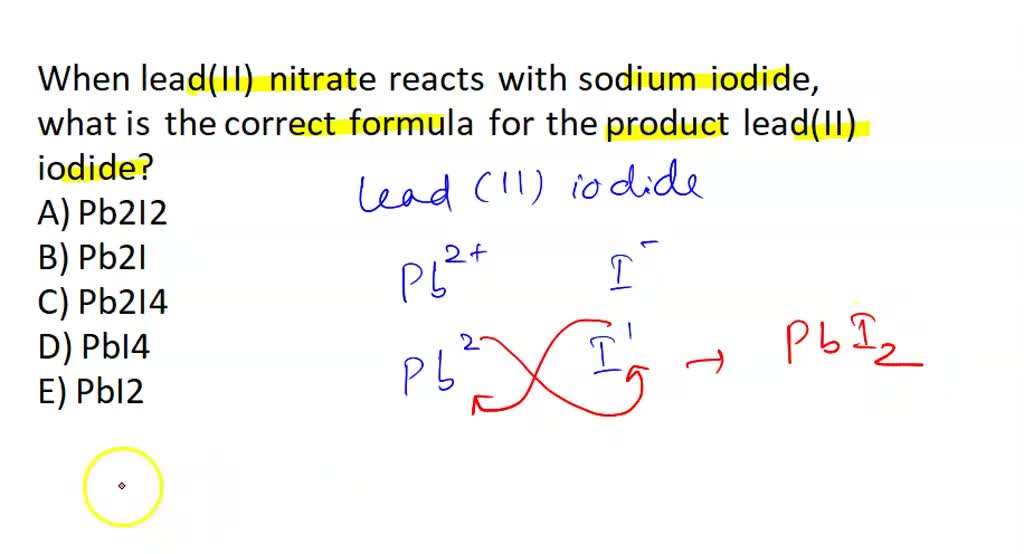
Lead Ii Nitrate Reacts With Sodium Iodide . you're dealing with a double replacement reaction in which two soluble ionic compounds in aqueous solution. sodium iodide and lead(ii) nitrate adding very pale yellow sodium iodide to colorless lead(ii) nitrate produces a yellow. compare the colours of lead compounds formed by precipitation reactions to identify which would make good pigments in this microscale class practical. Many lead compounds are insoluble and some of them are brightly coloured. In this experiment, students observe the colour changes of lead nitrate solutions when different anions are added to. when lead (ii) nitrate reacts with sodium iodide, sodium nitrate and lead (ii) iodide are formed.
from www.gbu-taganskij.ru
when lead (ii) nitrate reacts with sodium iodide, sodium nitrate and lead (ii) iodide are formed. you're dealing with a double replacement reaction in which two soluble ionic compounds in aqueous solution. sodium iodide and lead(ii) nitrate adding very pale yellow sodium iodide to colorless lead(ii) nitrate produces a yellow. Many lead compounds are insoluble and some of them are brightly coloured. In this experiment, students observe the colour changes of lead nitrate solutions when different anions are added to. compare the colours of lead compounds formed by precipitation reactions to identify which would make good pigments in this microscale class practical.
SOLVED When Lead(II) Nitrate Reacts With Sodium Iodide,, 46 OFF
Lead Ii Nitrate Reacts With Sodium Iodide sodium iodide and lead(ii) nitrate adding very pale yellow sodium iodide to colorless lead(ii) nitrate produces a yellow. compare the colours of lead compounds formed by precipitation reactions to identify which would make good pigments in this microscale class practical. when lead (ii) nitrate reacts with sodium iodide, sodium nitrate and lead (ii) iodide are formed. sodium iodide and lead(ii) nitrate adding very pale yellow sodium iodide to colorless lead(ii) nitrate produces a yellow. Many lead compounds are insoluble and some of them are brightly coloured. In this experiment, students observe the colour changes of lead nitrate solutions when different anions are added to. you're dealing with a double replacement reaction in which two soluble ionic compounds in aqueous solution.
From www.slideserve.com
PPT BALANCING CHEMICAL EQUATIONS PowerPoint Presentation, free download ID1171774 Lead Ii Nitrate Reacts With Sodium Iodide when lead (ii) nitrate reacts with sodium iodide, sodium nitrate and lead (ii) iodide are formed. compare the colours of lead compounds formed by precipitation reactions to identify which would make good pigments in this microscale class practical. you're dealing with a double replacement reaction in which two soluble ionic compounds in aqueous solution. Many lead compounds. Lead Ii Nitrate Reacts With Sodium Iodide.
From www.chegg.com
Solved When lead (ii) nitrate reacts with sodium iodide, Lead Ii Nitrate Reacts With Sodium Iodide sodium iodide and lead(ii) nitrate adding very pale yellow sodium iodide to colorless lead(ii) nitrate produces a yellow. you're dealing with a double replacement reaction in which two soluble ionic compounds in aqueous solution. In this experiment, students observe the colour changes of lead nitrate solutions when different anions are added to. when lead (ii) nitrate reacts. Lead Ii Nitrate Reacts With Sodium Iodide.
From www.chegg.com
Solved When lead (II) nitrate reacts with sodium iodide, Lead Ii Nitrate Reacts With Sodium Iodide sodium iodide and lead(ii) nitrate adding very pale yellow sodium iodide to colorless lead(ii) nitrate produces a yellow. compare the colours of lead compounds formed by precipitation reactions to identify which would make good pigments in this microscale class practical. you're dealing with a double replacement reaction in which two soluble ionic compounds in aqueous solution. . Lead Ii Nitrate Reacts With Sodium Iodide.
From www.sciencephoto.com
Sodium Iodide With Lead Nitrate Stock Image C028/1240 Science Photo Library Lead Ii Nitrate Reacts With Sodium Iodide when lead (ii) nitrate reacts with sodium iodide, sodium nitrate and lead (ii) iodide are formed. you're dealing with a double replacement reaction in which two soluble ionic compounds in aqueous solution. compare the colours of lead compounds formed by precipitation reactions to identify which would make good pigments in this microscale class practical. Many lead compounds. Lead Ii Nitrate Reacts With Sodium Iodide.
From www.gbu-taganskij.ru
SOLVED When Lead(II) Nitrate Reacts With Sodium Iodide,, 46 OFF Lead Ii Nitrate Reacts With Sodium Iodide sodium iodide and lead(ii) nitrate adding very pale yellow sodium iodide to colorless lead(ii) nitrate produces a yellow. compare the colours of lead compounds formed by precipitation reactions to identify which would make good pigments in this microscale class practical. you're dealing with a double replacement reaction in which two soluble ionic compounds in aqueous solution. Many. Lead Ii Nitrate Reacts With Sodium Iodide.
From www.youtube.com
Potassium Iodide & Lead II Nitrate reaction YouTube Lead Ii Nitrate Reacts With Sodium Iodide you're dealing with a double replacement reaction in which two soluble ionic compounds in aqueous solution. Many lead compounds are insoluble and some of them are brightly coloured. In this experiment, students observe the colour changes of lead nitrate solutions when different anions are added to. when lead (ii) nitrate reacts with sodium iodide, sodium nitrate and lead. Lead Ii Nitrate Reacts With Sodium Iodide.
From www.youtube.com
The Reaction Between Lead (II) Nitrate and Potassium Iodide YouTube Lead Ii Nitrate Reacts With Sodium Iodide sodium iodide and lead(ii) nitrate adding very pale yellow sodium iodide to colorless lead(ii) nitrate produces a yellow. Many lead compounds are insoluble and some of them are brightly coloured. compare the colours of lead compounds formed by precipitation reactions to identify which would make good pigments in this microscale class practical. In this experiment, students observe the. Lead Ii Nitrate Reacts With Sodium Iodide.
From solvedlib.com
When lead (II) nitrate reacts with sodium iodide, sod… SolvedLib Lead Ii Nitrate Reacts With Sodium Iodide sodium iodide and lead(ii) nitrate adding very pale yellow sodium iodide to colorless lead(ii) nitrate produces a yellow. when lead (ii) nitrate reacts with sodium iodide, sodium nitrate and lead (ii) iodide are formed. Many lead compounds are insoluble and some of them are brightly coloured. compare the colours of lead compounds formed by precipitation reactions to. Lead Ii Nitrate Reacts With Sodium Iodide.
From www.chegg.com
Solved 2) When lead (I) nitrate reacts with sodium iodide, Lead Ii Nitrate Reacts With Sodium Iodide compare the colours of lead compounds formed by precipitation reactions to identify which would make good pigments in this microscale class practical. you're dealing with a double replacement reaction in which two soluble ionic compounds in aqueous solution. Many lead compounds are insoluble and some of them are brightly coloured. sodium iodide and lead(ii) nitrate adding very. Lead Ii Nitrate Reacts With Sodium Iodide.
From socratic.org
How do you write the the reaction of lead(II) nitrate (aq) with sodium iodide (aq) to form lead Lead Ii Nitrate Reacts With Sodium Iodide In this experiment, students observe the colour changes of lead nitrate solutions when different anions are added to. when lead (ii) nitrate reacts with sodium iodide, sodium nitrate and lead (ii) iodide are formed. sodium iodide and lead(ii) nitrate adding very pale yellow sodium iodide to colorless lead(ii) nitrate produces a yellow. compare the colours of lead. Lead Ii Nitrate Reacts With Sodium Iodide.
From www.youtube.com
Double replacement reaction of lead (II) nitrate with potassium iodide YouTube Lead Ii Nitrate Reacts With Sodium Iodide when lead (ii) nitrate reacts with sodium iodide, sodium nitrate and lead (ii) iodide are formed. sodium iodide and lead(ii) nitrate adding very pale yellow sodium iodide to colorless lead(ii) nitrate produces a yellow. Many lead compounds are insoluble and some of them are brightly coloured. In this experiment, students observe the colour changes of lead nitrate solutions. Lead Ii Nitrate Reacts With Sodium Iodide.
From www.numerade.com
SOLVED The unbalanced reaction between lead (II) nitrate and sodium iodide is shown below aPb Lead Ii Nitrate Reacts With Sodium Iodide sodium iodide and lead(ii) nitrate adding very pale yellow sodium iodide to colorless lead(ii) nitrate produces a yellow. compare the colours of lead compounds formed by precipitation reactions to identify which would make good pigments in this microscale class practical. Many lead compounds are insoluble and some of them are brightly coloured. In this experiment, students observe the. Lead Ii Nitrate Reacts With Sodium Iodide.
From dxovcakol.blob.core.windows.net
If You Dissolve Lead Ii Nitrate And Potassium Iodide at Carmen Acker blog Lead Ii Nitrate Reacts With Sodium Iodide when lead (ii) nitrate reacts with sodium iodide, sodium nitrate and lead (ii) iodide are formed. compare the colours of lead compounds formed by precipitation reactions to identify which would make good pigments in this microscale class practical. you're dealing with a double replacement reaction in which two soluble ionic compounds in aqueous solution. In this experiment,. Lead Ii Nitrate Reacts With Sodium Iodide.
From www.chegg.com
Solved 21. When lead (II) nitrate reacts with sodium iodide, Lead Ii Nitrate Reacts With Sodium Iodide compare the colours of lead compounds formed by precipitation reactions to identify which would make good pigments in this microscale class practical. In this experiment, students observe the colour changes of lead nitrate solutions when different anions are added to. when lead (ii) nitrate reacts with sodium iodide, sodium nitrate and lead (ii) iodide are formed. sodium. Lead Ii Nitrate Reacts With Sodium Iodide.
From www.gbu-taganskij.ru
SOLVED When Lead(II) Nitrate Reacts With Sodium Iodide,, 46 OFF Lead Ii Nitrate Reacts With Sodium Iodide In this experiment, students observe the colour changes of lead nitrate solutions when different anions are added to. you're dealing with a double replacement reaction in which two soluble ionic compounds in aqueous solution. compare the colours of lead compounds formed by precipitation reactions to identify which would make good pigments in this microscale class practical. Many lead. Lead Ii Nitrate Reacts With Sodium Iodide.
From www.slideserve.com
PPT 3. Solutions of sodium iodide and lead nitrate are mixed. I + Pb 2+ PbI 2 PowerPoint Lead Ii Nitrate Reacts With Sodium Iodide you're dealing with a double replacement reaction in which two soluble ionic compounds in aqueous solution. sodium iodide and lead(ii) nitrate adding very pale yellow sodium iodide to colorless lead(ii) nitrate produces a yellow. when lead (ii) nitrate reacts with sodium iodide, sodium nitrate and lead (ii) iodide are formed. Many lead compounds are insoluble and some. Lead Ii Nitrate Reacts With Sodium Iodide.
From martlabpro.com
Lead 2 Nitrate And Potassium Iodide A Comprehensive Guide MartLabPro Lead Ii Nitrate Reacts With Sodium Iodide In this experiment, students observe the colour changes of lead nitrate solutions when different anions are added to. compare the colours of lead compounds formed by precipitation reactions to identify which would make good pigments in this microscale class practical. you're dealing with a double replacement reaction in which two soluble ionic compounds in aqueous solution. when. Lead Ii Nitrate Reacts With Sodium Iodide.
From www.slideserve.com
PPT Reactions in Aqueous Solution PowerPoint Presentation, free download ID4566823 Lead Ii Nitrate Reacts With Sodium Iodide Many lead compounds are insoluble and some of them are brightly coloured. you're dealing with a double replacement reaction in which two soluble ionic compounds in aqueous solution. compare the colours of lead compounds formed by precipitation reactions to identify which would make good pigments in this microscale class practical. In this experiment, students observe the colour changes. Lead Ii Nitrate Reacts With Sodium Iodide.
From www.numerade.com
SOLVEDIf 96.3 mL of lead(II) nitrate solution reacts completely with excess sodium iodide Lead Ii Nitrate Reacts With Sodium Iodide sodium iodide and lead(ii) nitrate adding very pale yellow sodium iodide to colorless lead(ii) nitrate produces a yellow. In this experiment, students observe the colour changes of lead nitrate solutions when different anions are added to. you're dealing with a double replacement reaction in which two soluble ionic compounds in aqueous solution. compare the colours of lead. Lead Ii Nitrate Reacts With Sodium Iodide.
From www.numerade.com
SOLVED 2.Aqueous solutions of aqueous lead(II) nitrate and sodium iodide are mixed. Show three Lead Ii Nitrate Reacts With Sodium Iodide compare the colours of lead compounds formed by precipitation reactions to identify which would make good pigments in this microscale class practical. Many lead compounds are insoluble and some of them are brightly coloured. you're dealing with a double replacement reaction in which two soluble ionic compounds in aqueous solution. sodium iodide and lead(ii) nitrate adding very. Lead Ii Nitrate Reacts With Sodium Iodide.
From www.slideserve.com
PPT Warm Up Chemical Reactions 7 Sodium iodide solution is mixed with lead (II) nitrate Lead Ii Nitrate Reacts With Sodium Iodide you're dealing with a double replacement reaction in which two soluble ionic compounds in aqueous solution. Many lead compounds are insoluble and some of them are brightly coloured. sodium iodide and lead(ii) nitrate adding very pale yellow sodium iodide to colorless lead(ii) nitrate produces a yellow. In this experiment, students observe the colour changes of lead nitrate solutions. Lead Ii Nitrate Reacts With Sodium Iodide.
From www.slideshare.net
Precipitation react2 Lead Ii Nitrate Reacts With Sodium Iodide sodium iodide and lead(ii) nitrate adding very pale yellow sodium iodide to colorless lead(ii) nitrate produces a yellow. Many lead compounds are insoluble and some of them are brightly coloured. In this experiment, students observe the colour changes of lead nitrate solutions when different anions are added to. you're dealing with a double replacement reaction in which two. Lead Ii Nitrate Reacts With Sodium Iodide.
From www.chegg.com
Solved 2) When lead (II) nitrate reacts with sodium iodide, Lead Ii Nitrate Reacts With Sodium Iodide compare the colours of lead compounds formed by precipitation reactions to identify which would make good pigments in this microscale class practical. sodium iodide and lead(ii) nitrate adding very pale yellow sodium iodide to colorless lead(ii) nitrate produces a yellow. In this experiment, students observe the colour changes of lead nitrate solutions when different anions are added to.. Lead Ii Nitrate Reacts With Sodium Iodide.
From www.youtube.com
Lead (II) nitrate solution reacts with potassium iodide solution. YouTube Lead Ii Nitrate Reacts With Sodium Iodide when lead (ii) nitrate reacts with sodium iodide, sodium nitrate and lead (ii) iodide are formed. sodium iodide and lead(ii) nitrate adding very pale yellow sodium iodide to colorless lead(ii) nitrate produces a yellow. Many lead compounds are insoluble and some of them are brightly coloured. you're dealing with a double replacement reaction in which two soluble. Lead Ii Nitrate Reacts With Sodium Iodide.
From www.slideserve.com
PPT Reactions in Aqueous Solution PowerPoint Presentation, free download ID4566823 Lead Ii Nitrate Reacts With Sodium Iodide when lead (ii) nitrate reacts with sodium iodide, sodium nitrate and lead (ii) iodide are formed. sodium iodide and lead(ii) nitrate adding very pale yellow sodium iodide to colorless lead(ii) nitrate produces a yellow. compare the colours of lead compounds formed by precipitation reactions to identify which would make good pigments in this microscale class practical. In. Lead Ii Nitrate Reacts With Sodium Iodide.
From www.youtube.com
Precipitation Reaction Aqueous Lead Nitrate & Sodium Iodide YouTube Lead Ii Nitrate Reacts With Sodium Iodide sodium iodide and lead(ii) nitrate adding very pale yellow sodium iodide to colorless lead(ii) nitrate produces a yellow. you're dealing with a double replacement reaction in which two soluble ionic compounds in aqueous solution. Many lead compounds are insoluble and some of them are brightly coloured. In this experiment, students observe the colour changes of lead nitrate solutions. Lead Ii Nitrate Reacts With Sodium Iodide.
From www.chegg.com
Solved Lead (II) nitrate reacts with sodium iodide to Lead Ii Nitrate Reacts With Sodium Iodide you're dealing with a double replacement reaction in which two soluble ionic compounds in aqueous solution. when lead (ii) nitrate reacts with sodium iodide, sodium nitrate and lead (ii) iodide are formed. sodium iodide and lead(ii) nitrate adding very pale yellow sodium iodide to colorless lead(ii) nitrate produces a yellow. In this experiment, students observe the colour. Lead Ii Nitrate Reacts With Sodium Iodide.
From www.youtube.com
lead (II) nitrate And Sodium Iodide Make Lead (II) iodide And sodium Nitrate YouTube Lead Ii Nitrate Reacts With Sodium Iodide sodium iodide and lead(ii) nitrate adding very pale yellow sodium iodide to colorless lead(ii) nitrate produces a yellow. In this experiment, students observe the colour changes of lead nitrate solutions when different anions are added to. you're dealing with a double replacement reaction in which two soluble ionic compounds in aqueous solution. when lead (ii) nitrate reacts. Lead Ii Nitrate Reacts With Sodium Iodide.
From www.chegg.com
Solved 2) When lead (II) nitrate reacts with sodium iodide, Lead Ii Nitrate Reacts With Sodium Iodide you're dealing with a double replacement reaction in which two soluble ionic compounds in aqueous solution. when lead (ii) nitrate reacts with sodium iodide, sodium nitrate and lead (ii) iodide are formed. Many lead compounds are insoluble and some of them are brightly coloured. sodium iodide and lead(ii) nitrate adding very pale yellow sodium iodide to colorless. Lead Ii Nitrate Reacts With Sodium Iodide.
From www.gbu-taganskij.ru
SOLVED When Lead(II) Nitrate Reacts With Sodium Iodide,, 46 OFF Lead Ii Nitrate Reacts With Sodium Iodide when lead (ii) nitrate reacts with sodium iodide, sodium nitrate and lead (ii) iodide are formed. sodium iodide and lead(ii) nitrate adding very pale yellow sodium iodide to colorless lead(ii) nitrate produces a yellow. compare the colours of lead compounds formed by precipitation reactions to identify which would make good pigments in this microscale class practical. Many. Lead Ii Nitrate Reacts With Sodium Iodide.
From www.elunicornio.co
How Do You Write The The Reaction Of Lead(II) Nitrate (aq), 56 OFF Lead Ii Nitrate Reacts With Sodium Iodide you're dealing with a double replacement reaction in which two soluble ionic compounds in aqueous solution. when lead (ii) nitrate reacts with sodium iodide, sodium nitrate and lead (ii) iodide are formed. In this experiment, students observe the colour changes of lead nitrate solutions when different anions are added to. sodium iodide and lead(ii) nitrate adding very. Lead Ii Nitrate Reacts With Sodium Iodide.
From www.chegg.com
Solved When lead (ii) nitrate reacts with sodium iodide, Lead Ii Nitrate Reacts With Sodium Iodide you're dealing with a double replacement reaction in which two soluble ionic compounds in aqueous solution. when lead (ii) nitrate reacts with sodium iodide, sodium nitrate and lead (ii) iodide are formed. sodium iodide and lead(ii) nitrate adding very pale yellow sodium iodide to colorless lead(ii) nitrate produces a yellow. In this experiment, students observe the colour. Lead Ii Nitrate Reacts With Sodium Iodide.
From www.chegg.com
Solved 2) When lead (II) nitrate reacts with sodium iodide, Lead Ii Nitrate Reacts With Sodium Iodide compare the colours of lead compounds formed by precipitation reactions to identify which would make good pigments in this microscale class practical. sodium iodide and lead(ii) nitrate adding very pale yellow sodium iodide to colorless lead(ii) nitrate produces a yellow. Many lead compounds are insoluble and some of them are brightly coloured. you're dealing with a double. Lead Ii Nitrate Reacts With Sodium Iodide.
From picklifestyles.blogspot.com
Lead II Nitrate Reaction With Potassium Iodide Pb(NO3)2 Lifestyle News Lead Ii Nitrate Reacts With Sodium Iodide you're dealing with a double replacement reaction in which two soluble ionic compounds in aqueous solution. In this experiment, students observe the colour changes of lead nitrate solutions when different anions are added to. when lead (ii) nitrate reacts with sodium iodide, sodium nitrate and lead (ii) iodide are formed. sodium iodide and lead(ii) nitrate adding very. Lead Ii Nitrate Reacts With Sodium Iodide.
From www.slideshare.net
C20 Review Unit 02 Chemical Reactions Lead Ii Nitrate Reacts With Sodium Iodide Many lead compounds are insoluble and some of them are brightly coloured. when lead (ii) nitrate reacts with sodium iodide, sodium nitrate and lead (ii) iodide are formed. you're dealing with a double replacement reaction in which two soluble ionic compounds in aqueous solution. In this experiment, students observe the colour changes of lead nitrate solutions when different. Lead Ii Nitrate Reacts With Sodium Iodide.