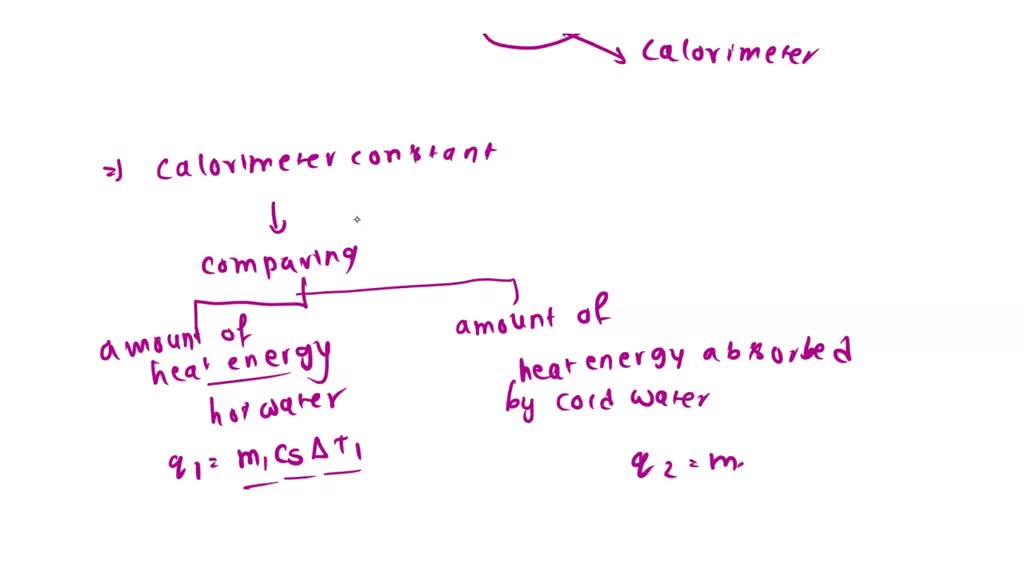
Can Calorimeter Constant Be Negative . Try performing multiple trials and averaging out the results of those trials to reduce. When 40.0 ml of water at 60.0 °c is added to 40.0 ml at 25.0 °c water already in a. Apply the first law of thermodynamics to calorimetry. How to calculate a calorimeter constant. The value of \(c\) is intrinsically a positive number, but \(δt\) and \(q\) can be either positive or negative, and they both must have the same. Compare heat flow from hot to cold objects in an ideal. If δ t and q are positive, then heat flows from the. The value of c is intrinsically a positive number, but δ t and q can be either positive or negative, and they both must have the same sign. The value of c is intrinsically a positive number, but δt and q can be either positive or negative, and they both must have the same sign.
from www.numerade.com
The value of c is intrinsically a positive number, but δt and q can be either positive or negative, and they both must have the same sign. The value of c is intrinsically a positive number, but δ t and q can be either positive or negative, and they both must have the same sign. Compare heat flow from hot to cold objects in an ideal. When 40.0 ml of water at 60.0 °c is added to 40.0 ml at 25.0 °c water already in a. Apply the first law of thermodynamics to calorimetry. Try performing multiple trials and averaging out the results of those trials to reduce. How to calculate a calorimeter constant. If δ t and q are positive, then heat flows from the. The value of \(c\) is intrinsically a positive number, but \(δt\) and \(q\) can be either positive or negative, and they both must have the same.
SOLVED Calorimetry Lab 1. In the measurement of a calorimeter constant, Ccal, a negative value
Can Calorimeter Constant Be Negative The value of c is intrinsically a positive number, but δ t and q can be either positive or negative, and they both must have the same sign. The value of c is intrinsically a positive number, but δ t and q can be either positive or negative, and they both must have the same sign. The value of \(c\) is intrinsically a positive number, but \(δt\) and \(q\) can be either positive or negative, and they both must have the same. Try performing multiple trials and averaging out the results of those trials to reduce. The value of c is intrinsically a positive number, but δt and q can be either positive or negative, and they both must have the same sign. Apply the first law of thermodynamics to calorimetry. Compare heat flow from hot to cold objects in an ideal. If δ t and q are positive, then heat flows from the. When 40.0 ml of water at 60.0 °c is added to 40.0 ml at 25.0 °c water already in a. How to calculate a calorimeter constant.
From www.numerade.com
SOLVEDAnalyeis Calculate the calorimeter constant (C) for your calorimeter; assuming the C of Can Calorimeter Constant Be Negative The value of \(c\) is intrinsically a positive number, but \(δt\) and \(q\) can be either positive or negative, and they both must have the same. Try performing multiple trials and averaging out the results of those trials to reduce. The value of c is intrinsically a positive number, but δ t and q can be either positive or negative,. Can Calorimeter Constant Be Negative.
From exourbxre.blob.core.windows.net
Why Is Calorimeter Constant Important at Waldman blog Can Calorimeter Constant Be Negative The value of c is intrinsically a positive number, but δt and q can be either positive or negative, and they both must have the same sign. Apply the first law of thermodynamics to calorimetry. Try performing multiple trials and averaging out the results of those trials to reduce. When 40.0 ml of water at 60.0 °c is added to. Can Calorimeter Constant Be Negative.
From www.numerade.com
SOLVED In the laboratory a "coffee cup" calorimeter, or constant pressure calorimeter, is Can Calorimeter Constant Be Negative The value of \(c\) is intrinsically a positive number, but \(δt\) and \(q\) can be either positive or negative, and they both must have the same. If δ t and q are positive, then heat flows from the. The value of c is intrinsically a positive number, but δt and q can be either positive or negative, and they both. Can Calorimeter Constant Be Negative.
From dxoazusse.blob.core.windows.net
Food Calorimetry Equation at Jesse Freeman blog Can Calorimeter Constant Be Negative If δ t and q are positive, then heat flows from the. Try performing multiple trials and averaging out the results of those trials to reduce. The value of \(c\) is intrinsically a positive number, but \(δt\) and \(q\) can be either positive or negative, and they both must have the same. Apply the first law of thermodynamics to calorimetry.. Can Calorimeter Constant Be Negative.
From exourbxre.blob.core.windows.net
Why Is Calorimeter Constant Important at Waldman blog Can Calorimeter Constant Be Negative Try performing multiple trials and averaging out the results of those trials to reduce. When 40.0 ml of water at 60.0 °c is added to 40.0 ml at 25.0 °c water already in a. The value of \(c\) is intrinsically a positive number, but \(δt\) and \(q\) can be either positive or negative, and they both must have the same.. Can Calorimeter Constant Be Negative.
From www.numerade.com
SOLVED When a solid dissolves in water; heat may be evolved or absorbed. The heat of Can Calorimeter Constant Be Negative How to calculate a calorimeter constant. When 40.0 ml of water at 60.0 °c is added to 40.0 ml at 25.0 °c water already in a. Apply the first law of thermodynamics to calorimetry. The value of c is intrinsically a positive number, but δ t and q can be either positive or negative, and they both must have the. Can Calorimeter Constant Be Negative.
From www.slideserve.com
PPT Thermochemistry PowerPoint Presentation, free download ID2692866 Can Calorimeter Constant Be Negative Try performing multiple trials and averaging out the results of those trials to reduce. If δ t and q are positive, then heat flows from the. Apply the first law of thermodynamics to calorimetry. When 40.0 ml of water at 60.0 °c is added to 40.0 ml at 25.0 °c water already in a. The value of \(c\) is intrinsically. Can Calorimeter Constant Be Negative.
From wisc.pb.unizin.org
Calorimetry continued Types of Calorimeters and Analyzing Heat Flow (M6Q5) UWMadison Can Calorimeter Constant Be Negative The value of c is intrinsically a positive number, but δt and q can be either positive or negative, and they both must have the same sign. The value of c is intrinsically a positive number, but δ t and q can be either positive or negative, and they both must have the same sign. The value of \(c\) is. Can Calorimeter Constant Be Negative.
From klakenjvx.blob.core.windows.net
Calculate Calorimeter Constant To 2 Significant Figures at Jesus Morris blog Can Calorimeter Constant Be Negative Apply the first law of thermodynamics to calorimetry. If δ t and q are positive, then heat flows from the. The value of \(c\) is intrinsically a positive number, but \(δt\) and \(q\) can be either positive or negative, and they both must have the same. When 40.0 ml of water at 60.0 °c is added to 40.0 ml at. Can Calorimeter Constant Be Negative.
From www.numerade.com
SOLVED Calorimetry Lab 1. In the measurement of a calorimeter constant, Ccal, a negative value Can Calorimeter Constant Be Negative When 40.0 ml of water at 60.0 °c is added to 40.0 ml at 25.0 °c water already in a. If δ t and q are positive, then heat flows from the. Compare heat flow from hot to cold objects in an ideal. How to calculate a calorimeter constant. Try performing multiple trials and averaging out the results of those. Can Calorimeter Constant Be Negative.
From www.tessshebaylo.com
Equation For Constant Volume Calorimetry Tessshebaylo Can Calorimeter Constant Be Negative When 40.0 ml of water at 60.0 °c is added to 40.0 ml at 25.0 °c water already in a. The value of c is intrinsically a positive number, but δt and q can be either positive or negative, and they both must have the same sign. Try performing multiple trials and averaging out the results of those trials to. Can Calorimeter Constant Be Negative.
From grade12uchemistry.weebly.com
Calorimetry Grade12UChemistry Can Calorimeter Constant Be Negative If δ t and q are positive, then heat flows from the. The value of c is intrinsically a positive number, but δt and q can be either positive or negative, and they both must have the same sign. When 40.0 ml of water at 60.0 °c is added to 40.0 ml at 25.0 °c water already in a. The. Can Calorimeter Constant Be Negative.
From www.slideserve.com
PPT Zumdahl Chapter 6 PowerPoint Presentation, free download ID4269919 Can Calorimeter Constant Be Negative Try performing multiple trials and averaging out the results of those trials to reduce. When 40.0 ml of water at 60.0 °c is added to 40.0 ml at 25.0 °c water already in a. The value of c is intrinsically a positive number, but δt and q can be either positive or negative, and they both must have the same. Can Calorimeter Constant Be Negative.
From exourbxre.blob.core.windows.net
Why Is Calorimeter Constant Important at Waldman blog Can Calorimeter Constant Be Negative Try performing multiple trials and averaging out the results of those trials to reduce. If δ t and q are positive, then heat flows from the. The value of c is intrinsically a positive number, but δt and q can be either positive or negative, and they both must have the same sign. How to calculate a calorimeter constant. The. Can Calorimeter Constant Be Negative.
From www.slideserve.com
PPT Heating Curves PowerPoint Presentation, free download ID4576403 Can Calorimeter Constant Be Negative The value of c is intrinsically a positive number, but δ t and q can be either positive or negative, and they both must have the same sign. Try performing multiple trials and averaging out the results of those trials to reduce. When 40.0 ml of water at 60.0 °c is added to 40.0 ml at 25.0 °c water already. Can Calorimeter Constant Be Negative.
From quizdbbarefooted.z21.web.core.windows.net
How To Calculate Calorimeter Can Calorimeter Constant Be Negative The value of c is intrinsically a positive number, but δ t and q can be either positive or negative, and they both must have the same sign. The value of \(c\) is intrinsically a positive number, but \(δt\) and \(q\) can be either positive or negative, and they both must have the same. The value of c is intrinsically. Can Calorimeter Constant Be Negative.
From www.chegg.com
Solved A bomb calorimeter, or constant volume calorimeter, Can Calorimeter Constant Be Negative When 40.0 ml of water at 60.0 °c is added to 40.0 ml at 25.0 °c water already in a. The value of \(c\) is intrinsically a positive number, but \(δt\) and \(q\) can be either positive or negative, and they both must have the same. If δ t and q are positive, then heat flows from the. The value. Can Calorimeter Constant Be Negative.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation ID1609320 Can Calorimeter Constant Be Negative Compare heat flow from hot to cold objects in an ideal. The value of c is intrinsically a positive number, but δt and q can be either positive or negative, and they both must have the same sign. Apply the first law of thermodynamics to calorimetry. If δ t and q are positive, then heat flows from the. Try performing. Can Calorimeter Constant Be Negative.
From klakenjvx.blob.core.windows.net
Calculate Calorimeter Constant To 2 Significant Figures at Jesus Morris blog Can Calorimeter Constant Be Negative Apply the first law of thermodynamics to calorimetry. The value of c is intrinsically a positive number, but δ t and q can be either positive or negative, and they both must have the same sign. How to calculate a calorimeter constant. The value of c is intrinsically a positive number, but δt and q can be either positive or. Can Calorimeter Constant Be Negative.
From www.reddit.com
Calculating calorimeter constant is coming out negative and don’t know why r/chemhelp Can Calorimeter Constant Be Negative The value of c is intrinsically a positive number, but δt and q can be either positive or negative, and they both must have the same sign. How to calculate a calorimeter constant. Apply the first law of thermodynamics to calorimetry. If δ t and q are positive, then heat flows from the. The value of c is intrinsically a. Can Calorimeter Constant Be Negative.
From slideplayer.com
Chapter 17 Thermochemistry 17.2 Measuring and Expressing ppt download Can Calorimeter Constant Be Negative If δ t and q are positive, then heat flows from the. Apply the first law of thermodynamics to calorimetry. How to calculate a calorimeter constant. The value of c is intrinsically a positive number, but δ t and q can be either positive or negative, and they both must have the same sign. Try performing multiple trials and averaging. Can Calorimeter Constant Be Negative.
From wisc.pb.unizin.org
Calorimetry continued Types of Calorimeters and Analyzing Heat Flow (M6Q5) UWMadison Can Calorimeter Constant Be Negative Apply the first law of thermodynamics to calorimetry. Try performing multiple trials and averaging out the results of those trials to reduce. The value of \(c\) is intrinsically a positive number, but \(δt\) and \(q\) can be either positive or negative, and they both must have the same. How to calculate a calorimeter constant. When 40.0 ml of water at. Can Calorimeter Constant Be Negative.
From www.chegg.com
Solved Constantpressure calorimeters can be calibrated by Can Calorimeter Constant Be Negative How to calculate a calorimeter constant. The value of c is intrinsically a positive number, but δt and q can be either positive or negative, and they both must have the same sign. The value of c is intrinsically a positive number, but δ t and q can be either positive or negative, and they both must have the same. Can Calorimeter Constant Be Negative.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation, free download ID373573 Can Calorimeter Constant Be Negative How to calculate a calorimeter constant. Compare heat flow from hot to cold objects in an ideal. When 40.0 ml of water at 60.0 °c is added to 40.0 ml at 25.0 °c water already in a. If δ t and q are positive, then heat flows from the. Try performing multiple trials and averaging out the results of those. Can Calorimeter Constant Be Negative.
From courses.lumenlearning.com
Calorimetry Chemistry for Majors Can Calorimeter Constant Be Negative If δ t and q are positive, then heat flows from the. Try performing multiple trials and averaging out the results of those trials to reduce. How to calculate a calorimeter constant. The value of c is intrinsically a positive number, but δ t and q can be either positive or negative, and they both must have the same sign.. Can Calorimeter Constant Be Negative.
From www.grc.nasa.gov
Heat Transfer Can Calorimeter Constant Be Negative Compare heat flow from hot to cold objects in an ideal. How to calculate a calorimeter constant. Apply the first law of thermodynamics to calorimetry. If δ t and q are positive, then heat flows from the. When 40.0 ml of water at 60.0 °c is added to 40.0 ml at 25.0 °c water already in a. The value of. Can Calorimeter Constant Be Negative.
From www.youtube.com
Thermochemistry 5 Determining Calorimeter Constant 6m16s YouTube Can Calorimeter Constant Be Negative If δ t and q are positive, then heat flows from the. How to calculate a calorimeter constant. The value of c is intrinsically a positive number, but δt and q can be either positive or negative, and they both must have the same sign. The value of \(c\) is intrinsically a positive number, but \(δt\) and \(q\) can be. Can Calorimeter Constant Be Negative.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation, free download ID3850751 Can Calorimeter Constant Be Negative Try performing multiple trials and averaging out the results of those trials to reduce. If δ t and q are positive, then heat flows from the. The value of c is intrinsically a positive number, but δ t and q can be either positive or negative, and they both must have the same sign. Apply the first law of thermodynamics. Can Calorimeter Constant Be Negative.
From cernvbyt.blob.core.windows.net
Can Heat Capacity Of Calorimeter Be Negative at Lulu Seaton blog Can Calorimeter Constant Be Negative The value of c is intrinsically a positive number, but δ t and q can be either positive or negative, and they both must have the same sign. How to calculate a calorimeter constant. When 40.0 ml of water at 60.0 °c is added to 40.0 ml at 25.0 °c water already in a. The value of c is intrinsically. Can Calorimeter Constant Be Negative.
From www.youtube.com
Calorimetry, Bomb Calorimetry, Constant Pressure Calorimetry FULL Review for Exams YouTube Can Calorimeter Constant Be Negative When 40.0 ml of water at 60.0 °c is added to 40.0 ml at 25.0 °c water already in a. The value of \(c\) is intrinsically a positive number, but \(δt\) and \(q\) can be either positive or negative, and they both must have the same. If δ t and q are positive, then heat flows from the. Compare heat. Can Calorimeter Constant Be Negative.
From www.slideserve.com
PPT Chapter 5 Thermochemistry PowerPoint Presentation, free download ID225035 Can Calorimeter Constant Be Negative Apply the first law of thermodynamics to calorimetry. The value of c is intrinsically a positive number, but δt and q can be either positive or negative, and they both must have the same sign. Try performing multiple trials and averaging out the results of those trials to reduce. If δ t and q are positive, then heat flows from. Can Calorimeter Constant Be Negative.
From www.youtube.com
Using Calorimetry to Calculate Enthalpies of Reaction Chemistry Tutorial YouTube Can Calorimeter Constant Be Negative How to calculate a calorimeter constant. Try performing multiple trials and averaging out the results of those trials to reduce. The value of \(c\) is intrinsically a positive number, but \(δt\) and \(q\) can be either positive or negative, and they both must have the same. The value of c is intrinsically a positive number, but δt and q can. Can Calorimeter Constant Be Negative.
From joiolgume.blob.core.windows.net
Calorimetry Heat Calculations at Brenda Salzer blog Can Calorimeter Constant Be Negative How to calculate a calorimeter constant. The value of c is intrinsically a positive number, but δt and q can be either positive or negative, and they both must have the same sign. When 40.0 ml of water at 60.0 °c is added to 40.0 ml at 25.0 °c water already in a. Try performing multiple trials and averaging out. Can Calorimeter Constant Be Negative.
From www.chegg.com
Solved In the laboratory a "coffee cup" calorimeter, or Can Calorimeter Constant Be Negative The value of c is intrinsically a positive number, but δ t and q can be either positive or negative, and they both must have the same sign. Try performing multiple trials and averaging out the results of those trials to reduce. Compare heat flow from hot to cold objects in an ideal. If δ t and q are positive,. Can Calorimeter Constant Be Negative.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation, free download ID1875569 Can Calorimeter Constant Be Negative Apply the first law of thermodynamics to calorimetry. If δ t and q are positive, then heat flows from the. Compare heat flow from hot to cold objects in an ideal. The value of c is intrinsically a positive number, but δ t and q can be either positive or negative, and they both must have the same sign. How. Can Calorimeter Constant Be Negative.