Standard Enthalpy Of Formation Is Zero For O2 . To find the δh reaction o, use the formula for the standard enthalpy change of formation: Since oxygen is an element in its standard state, its enthalpy of formation is zero. The standard enthalpy of formation of any element in its standard state is zero by definition. \[\delta h_{reaction}^o = \sum {\delta. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. For example, although oxygen can exist as ozone (o 3 ), atomic oxygen (o), and. The same is true other other gaseous elements, such as hydrogen and nitrogen, and solid elements, such as carbon in its. Doing the math gives us δh comb o = −1367 kj/mol of ethyl. By definition, the standard enthalpy of formation of an element in its most stable form is equal to zero under standard conditions, which is 1 atm for. Oxygen (the element) at standard state is o 2. A standard enthalpy of formation describes the change in enthalpy during the formation of 1 mol of a target compound by.
from www.numerade.com
193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. Doing the math gives us δh comb o = −1367 kj/mol of ethyl. By definition, the standard enthalpy of formation of an element in its most stable form is equal to zero under standard conditions, which is 1 atm for. For example, although oxygen can exist as ozone (o 3 ), atomic oxygen (o), and. \[\delta h_{reaction}^o = \sum {\delta. To find the δh reaction o, use the formula for the standard enthalpy change of formation: The same is true other other gaseous elements, such as hydrogen and nitrogen, and solid elements, such as carbon in its. Oxygen (the element) at standard state is o 2. The standard enthalpy of formation of any element in its standard state is zero by definition. Since oxygen is an element in its standard state, its enthalpy of formation is zero.
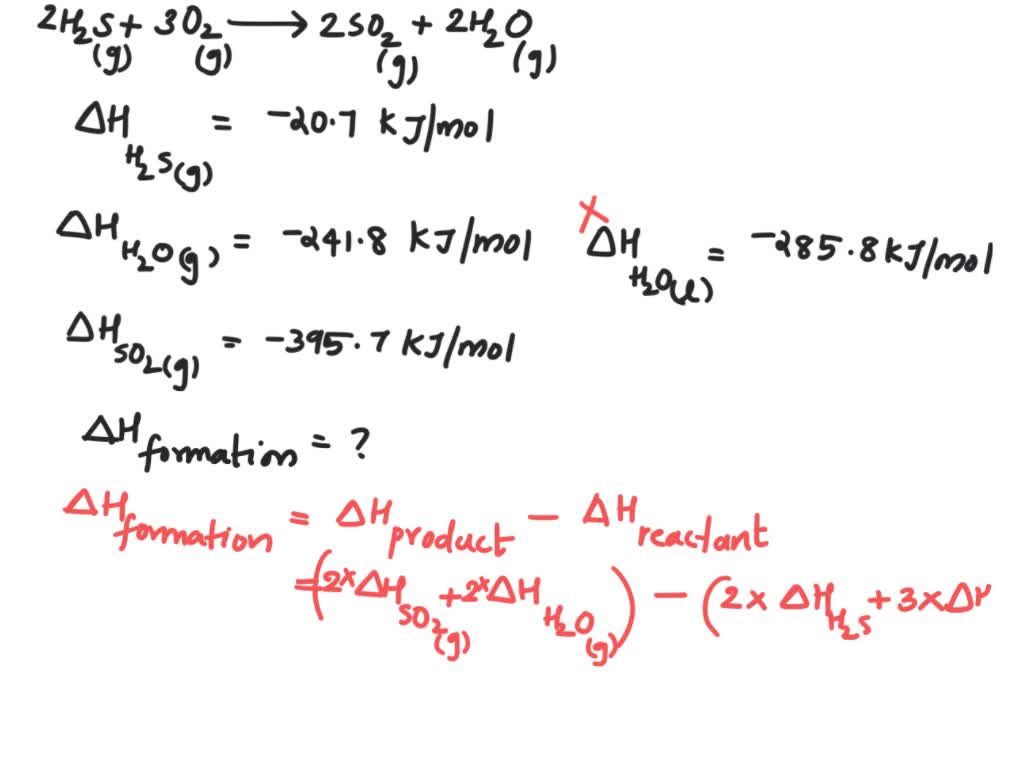
Consider the following reaction 2 H2S (g) + 3 O2 (g) → 2 SO2 (g) + 2 H2O (g) Calculate the
Standard Enthalpy Of Formation Is Zero For O2 Doing the math gives us δh comb o = −1367 kj/mol of ethyl. The same is true other other gaseous elements, such as hydrogen and nitrogen, and solid elements, such as carbon in its. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. Since oxygen is an element in its standard state, its enthalpy of formation is zero. Oxygen (the element) at standard state is o 2. Doing the math gives us δh comb o = −1367 kj/mol of ethyl. The standard enthalpy of formation of any element in its standard state is zero by definition. A standard enthalpy of formation describes the change in enthalpy during the formation of 1 mol of a target compound by. To find the δh reaction o, use the formula for the standard enthalpy change of formation: By definition, the standard enthalpy of formation of an element in its most stable form is equal to zero under standard conditions, which is 1 atm for. \[\delta h_{reaction}^o = \sum {\delta. For example, although oxygen can exist as ozone (o 3 ), atomic oxygen (o), and.
From www.toppr.com
Formation of ozone from oxygen is an endothermic process. In the upper atmosphere, ultraviolet Standard Enthalpy Of Formation Is Zero For O2 A standard enthalpy of formation describes the change in enthalpy during the formation of 1 mol of a target compound by. Oxygen (the element) at standard state is o 2. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. The same is true other other gaseous. Standard Enthalpy Of Formation Is Zero For O2.
From schoolworkhelper.net
Standard Enthalpies of Formation SchoolWorkHelper Standard Enthalpy Of Formation Is Zero For O2 Since oxygen is an element in its standard state, its enthalpy of formation is zero. \[\delta h_{reaction}^o = \sum {\delta. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. A standard enthalpy of formation describes the change in enthalpy during the formation of 1 mol of. Standard Enthalpy Of Formation Is Zero For O2.
From printablevascelomgm.z13.web.core.windows.net
How To Determine The Heat Of Formation Standard Enthalpy Of Formation Is Zero For O2 By definition, the standard enthalpy of formation of an element in its most stable form is equal to zero under standard conditions, which is 1 atm for. \[\delta h_{reaction}^o = \sum {\delta. A standard enthalpy of formation describes the change in enthalpy during the formation of 1 mol of a target compound by. Doing the math gives us δh comb. Standard Enthalpy Of Formation Is Zero For O2.
From www.thoughtco.com
Why the Standard Enthalpy of Formation of O2 Equals Zero Standard Enthalpy Of Formation Is Zero For O2 Since oxygen is an element in its standard state, its enthalpy of formation is zero. By definition, the standard enthalpy of formation of an element in its most stable form is equal to zero under standard conditions, which is 1 atm for. The same is true other other gaseous elements, such as hydrogen and nitrogen, and solid elements, such as. Standard Enthalpy Of Formation Is Zero For O2.
From www.toppr.com
(i) The enthalpy of reaction the reaction 2H2 (g) + O2 (g) → 2H20 (1) is A,H=572 kJ What will Standard Enthalpy Of Formation Is Zero For O2 \[\delta h_{reaction}^o = \sum {\delta. Doing the math gives us δh comb o = −1367 kj/mol of ethyl. Since oxygen is an element in its standard state, its enthalpy of formation is zero. By definition, the standard enthalpy of formation of an element in its most stable form is equal to zero under standard conditions, which is 1 atm for.. Standard Enthalpy Of Formation Is Zero For O2.
From cewuaeqb.blob.core.windows.net
How Do You Work Out Standard Enthalpy Of Formation at Connie Stroud blog Standard Enthalpy Of Formation Is Zero For O2 Doing the math gives us δh comb o = −1367 kj/mol of ethyl. For example, although oxygen can exist as ozone (o 3 ), atomic oxygen (o), and. Since oxygen is an element in its standard state, its enthalpy of formation is zero. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of. Standard Enthalpy Of Formation Is Zero For O2.
From www.researchgate.net
The standard free enthalpy DG 0 of the oxygen vacancy formation as a... Download Scientific Standard Enthalpy Of Formation Is Zero For O2 For example, although oxygen can exist as ozone (o 3 ), atomic oxygen (o), and. Since oxygen is an element in its standard state, its enthalpy of formation is zero. Oxygen (the element) at standard state is o 2. The standard enthalpy of formation of any element in its standard state is zero by definition. \[\delta h_{reaction}^o = \sum {\delta.. Standard Enthalpy Of Formation Is Zero For O2.
From www.numerade.com
SOLVED . Calculate the standard enthalpy of formation of dinitrogen pentoxide from the Standard Enthalpy Of Formation Is Zero For O2 For example, although oxygen can exist as ozone (o 3 ), atomic oxygen (o), and. The standard enthalpy of formation of any element in its standard state is zero by definition. A standard enthalpy of formation describes the change in enthalpy during the formation of 1 mol of a target compound by. Since oxygen is an element in its standard. Standard Enthalpy Of Formation Is Zero For O2.
From www.chegg.com
Solved Use a standard enthalpies of formation table to Standard Enthalpy Of Formation Is Zero For O2 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. For example, although oxygen can exist as ozone (o 3 ), atomic oxygen (o), and. To find the δh reaction o, use the formula for the standard enthalpy change of formation: The same is true other other. Standard Enthalpy Of Formation Is Zero For O2.
From www.slideserve.com
PPT THERMOCHEMISTRY PowerPoint Presentation, free download ID5773812 Standard Enthalpy Of Formation Is Zero For O2 The same is true other other gaseous elements, such as hydrogen and nitrogen, and solid elements, such as carbon in its. A standard enthalpy of formation describes the change in enthalpy during the formation of 1 mol of a target compound by. Doing the math gives us δh comb o = −1367 kj/mol of ethyl. \[\delta h_{reaction}^o = \sum {\delta.. Standard Enthalpy Of Formation Is Zero For O2.
From askfilo.com
The standard enthalpy of formation of oxygen gas is Filo Standard Enthalpy Of Formation Is Zero For O2 By definition, the standard enthalpy of formation of an element in its most stable form is equal to zero under standard conditions, which is 1 atm for. To find the δh reaction o, use the formula for the standard enthalpy change of formation: \[\delta h_{reaction}^o = \sum {\delta. A standard enthalpy of formation describes the change in enthalpy during the. Standard Enthalpy Of Formation Is Zero For O2.
From studylib.net
I. Standard Enthalpies of Formation Standard Enthalpy Of Formation Is Zero For O2 The standard enthalpy of formation of any element in its standard state is zero by definition. The same is true other other gaseous elements, such as hydrogen and nitrogen, and solid elements, such as carbon in its. A standard enthalpy of formation describes the change in enthalpy during the formation of 1 mol of a target compound by. \[\delta h_{reaction}^o. Standard Enthalpy Of Formation Is Zero For O2.
From www.youtube.com
CHEMISTRY 101 Standard enthalpies of formation and reaction YouTube Standard Enthalpy Of Formation Is Zero For O2 Since oxygen is an element in its standard state, its enthalpy of formation is zero. \[\delta h_{reaction}^o = \sum {\delta. A standard enthalpy of formation describes the change in enthalpy during the formation of 1 mol of a target compound by. Doing the math gives us δh comb o = −1367 kj/mol of ethyl. The same is true other other. Standard Enthalpy Of Formation Is Zero For O2.
From www.slideshare.net
Standard enthalpy of formation Standard Enthalpy Of Formation Is Zero For O2 The same is true other other gaseous elements, such as hydrogen and nitrogen, and solid elements, such as carbon in its. To find the δh reaction o, use the formula for the standard enthalpy change of formation: A standard enthalpy of formation describes the change in enthalpy during the formation of 1 mol of a target compound by. By definition,. Standard Enthalpy Of Formation Is Zero For O2.
From www.numerade.com
SOLVED Use the standard enthalpy of formation (ΔHf) values in Appendix I to calculate the Standard Enthalpy Of Formation Is Zero For O2 The same is true other other gaseous elements, such as hydrogen and nitrogen, and solid elements, such as carbon in its. Doing the math gives us δh comb o = −1367 kj/mol of ethyl. To find the δh reaction o, use the formula for the standard enthalpy change of formation: By definition, the standard enthalpy of formation of an element. Standard Enthalpy Of Formation Is Zero For O2.
From cepbtpfh.blob.core.windows.net
Standard Heat Of Formation Hydrogen Peroxide at John Ahmed blog Standard Enthalpy Of Formation Is Zero For O2 Since oxygen is an element in its standard state, its enthalpy of formation is zero. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. Oxygen (the element) at standard state is o 2. For example, although oxygen can exist as ozone (o 3 ), atomic oxygen. Standard Enthalpy Of Formation Is Zero For O2.
From www.numerade.com
SOLVEDFor which reaction the change of the enthalpy of the reaction (4H?) exactly equals the Standard Enthalpy Of Formation Is Zero For O2 Doing the math gives us δh comb o = −1367 kj/mol of ethyl. For example, although oxygen can exist as ozone (o 3 ), atomic oxygen (o), and. The standard enthalpy of formation of any element in its standard state is zero by definition. \[\delta h_{reaction}^o = \sum {\delta. The same is true other other gaseous elements, such as hydrogen. Standard Enthalpy Of Formation Is Zero For O2.
From cewuaeqb.blob.core.windows.net
How Do You Work Out Standard Enthalpy Of Formation at Connie Stroud blog Standard Enthalpy Of Formation Is Zero For O2 A standard enthalpy of formation describes the change in enthalpy during the formation of 1 mol of a target compound by. For example, although oxygen can exist as ozone (o 3 ), atomic oxygen (o), and. To find the δh reaction o, use the formula for the standard enthalpy change of formation: Doing the math gives us δh comb o. Standard Enthalpy Of Formation Is Zero For O2.
From slidesharetrick.blogspot.com
O2 Enthalpy Of Formation slidesharetrick Standard Enthalpy Of Formation Is Zero For O2 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. A standard enthalpy of formation describes the change in enthalpy during the formation of 1 mol of a target compound by. For example, although oxygen can exist as ozone (o 3 ), atomic oxygen (o), and. Since. Standard Enthalpy Of Formation Is Zero For O2.
From www.chemistryspace.com
Standard Enthalpy of Formation Standard Enthalpy Of Formation Is Zero For O2 Since oxygen is an element in its standard state, its enthalpy of formation is zero. Oxygen (the element) at standard state is o 2. \[\delta h_{reaction}^o = \sum {\delta. The same is true other other gaseous elements, such as hydrogen and nitrogen, and solid elements, such as carbon in its. A standard enthalpy of formation describes the change in enthalpy. Standard Enthalpy Of Formation Is Zero For O2.
From www.numerade.com
SOLVED Hess' Law questions Q1. Using the data in the table below, calculate the standard Standard Enthalpy Of Formation Is Zero For O2 The same is true other other gaseous elements, such as hydrogen and nitrogen, and solid elements, such as carbon in its. For example, although oxygen can exist as ozone (o 3 ), atomic oxygen (o), and. A standard enthalpy of formation describes the change in enthalpy during the formation of 1 mol of a target compound by. Oxygen (the element). Standard Enthalpy Of Formation Is Zero For O2.
From narodnatribuna.info
Calculating Reaction Enthalpy From Enthalpies Of Formation Standard Enthalpy Of Formation Is Zero For O2 A standard enthalpy of formation describes the change in enthalpy during the formation of 1 mol of a target compound by. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. Doing the math gives us δh comb o = −1367 kj/mol of ethyl. Oxygen (the element). Standard Enthalpy Of Formation Is Zero For O2.
From www.numerade.com
SOLVED Calculate the standard enthalpy of formation and the standard enthalpy change, both at Standard Enthalpy Of Formation Is Zero For O2 A standard enthalpy of formation describes the change in enthalpy during the formation of 1 mol of a target compound by. Since oxygen is an element in its standard state, its enthalpy of formation is zero. \[\delta h_{reaction}^o = \sum {\delta. Oxygen (the element) at standard state is o 2. Doing the math gives us δh comb o = −1367. Standard Enthalpy Of Formation Is Zero For O2.
From oxygengasnaraeru.blogspot.com
Oxygen Gas Enthalpy Of Formation Of Oxygen Gas Standard Enthalpy Of Formation Is Zero For O2 The standard enthalpy of formation of any element in its standard state is zero by definition. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. For example, although oxygen can exist as ozone (o 3 ), atomic oxygen (o), and. A standard enthalpy of formation describes. Standard Enthalpy Of Formation Is Zero For O2.
From joilylugg.blob.core.windows.net
Standard Enthalpy Of Formation Def at Sandra Leonard blog Standard Enthalpy Of Formation Is Zero For O2 By definition, the standard enthalpy of formation of an element in its most stable form is equal to zero under standard conditions, which is 1 atm for. The standard enthalpy of formation of any element in its standard state is zero by definition. A standard enthalpy of formation describes the change in enthalpy during the formation of 1 mol of. Standard Enthalpy Of Formation Is Zero For O2.
From www.numerade.com
SOLVED Using Standard Enthalpy of Formation Enthalpy Test (all answers to three sig figs Standard Enthalpy Of Formation Is Zero For O2 \[\delta h_{reaction}^o = \sum {\delta. The same is true other other gaseous elements, such as hydrogen and nitrogen, and solid elements, such as carbon in its. A standard enthalpy of formation describes the change in enthalpy during the formation of 1 mol of a target compound by. The standard enthalpy of formation of any element in its standard state is. Standard Enthalpy Of Formation Is Zero For O2.
From www.slideserve.com
PPT STANDARD MOLAR ENTHALPY OF FORMATION PowerPoint Presentation, free download ID2964088 Standard Enthalpy Of Formation Is Zero For O2 \[\delta h_{reaction}^o = \sum {\delta. A standard enthalpy of formation describes the change in enthalpy during the formation of 1 mol of a target compound by. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. Oxygen (the element) at standard state is o 2. For example,. Standard Enthalpy Of Formation Is Zero For O2.
From classnotes.org.in
Enthalpies Of Reaction Chemistry, Class 11, Thermodynamics Standard Enthalpy Of Formation Is Zero For O2 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. To find the δh reaction o, use the formula for the standard enthalpy change of formation: The standard enthalpy of formation of any element in its standard state is zero by definition. For example, although oxygen can. Standard Enthalpy Of Formation Is Zero For O2.
From www.numerade.com
Consider the following reaction 2 H2S (g) + 3 O2 (g) → 2 SO2 (g) + 2 H2O (g) Calculate the Standard Enthalpy Of Formation Is Zero For O2 The standard enthalpy of formation of any element in its standard state is zero by definition. Oxygen (the element) at standard state is o 2. The same is true other other gaseous elements, such as hydrogen and nitrogen, and solid elements, such as carbon in its. By definition, the standard enthalpy of formation of an element in its most stable. Standard Enthalpy Of Formation Is Zero For O2.
From www.slideserve.com
PPT Chapter 15 Standard enthalpy change of a reaction PowerPoint Presentation ID5860500 Standard Enthalpy Of Formation Is Zero For O2 By definition, the standard enthalpy of formation of an element in its most stable form is equal to zero under standard conditions, which is 1 atm for. The same is true other other gaseous elements, such as hydrogen and nitrogen, and solid elements, such as carbon in its. For example, although oxygen can exist as ozone (o 3 ), atomic. Standard Enthalpy Of Formation Is Zero For O2.
From www.slideshare.net
Tang 03 enthalpy of formation and combustion Standard Enthalpy Of Formation Is Zero For O2 The standard enthalpy of formation of any element in its standard state is zero by definition. A standard enthalpy of formation describes the change in enthalpy during the formation of 1 mol of a target compound by. Oxygen (the element) at standard state is o 2. The same is true other other gaseous elements, such as hydrogen and nitrogen, and. Standard Enthalpy Of Formation Is Zero For O2.
From pdfprof.com
PDF Télécharger enthalpie standard de formation o2 Gratuit PDF Standard Enthalpy Of Formation Is Zero For O2 Since oxygen is an element in its standard state, its enthalpy of formation is zero. By definition, the standard enthalpy of formation of an element in its most stable form is equal to zero under standard conditions, which is 1 atm for. \[\delta h_{reaction}^o = \sum {\delta. To find the δh reaction o, use the formula for the standard enthalpy. Standard Enthalpy Of Formation Is Zero For O2.
From www.slideserve.com
PPT Standard Enthalpies of Formation PowerPoint Presentation, free download ID4097208 Standard Enthalpy Of Formation Is Zero For O2 By definition, the standard enthalpy of formation of an element in its most stable form is equal to zero under standard conditions, which is 1 atm for. The standard enthalpy of formation of any element in its standard state is zero by definition. For example, although oxygen can exist as ozone (o 3 ), atomic oxygen (o), and. Since oxygen. Standard Enthalpy Of Formation Is Zero For O2.
From www.chem.fsu.edu
CHM1045 Enthalpy Lecture Standard Enthalpy Of Formation Is Zero For O2 The same is true other other gaseous elements, such as hydrogen and nitrogen, and solid elements, such as carbon in its. To find the δh reaction o, use the formula for the standard enthalpy change of formation: Oxygen (the element) at standard state is o 2. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat. Standard Enthalpy Of Formation Is Zero For O2.
From www.youtube.com
CHEM 101 Using Standard Enthalpies of Formation and Standard Enthalpy Change YouTube Standard Enthalpy Of Formation Is Zero For O2 Doing the math gives us δh comb o = −1367 kj/mol of ethyl. A standard enthalpy of formation describes the change in enthalpy during the formation of 1 mol of a target compound by. For example, although oxygen can exist as ozone (o 3 ), atomic oxygen (o), and. To find the δh reaction o, use the formula for the. Standard Enthalpy Of Formation Is Zero For O2.