Water Boiling Isobaric Process . An isobaric process in action. When the volume of a system remains constant during a thermodynamic process, the process is called isochoric. As the vessel is open, the pressure of the system will neither increase nor decrease. A quintessential example of an isobaric process is boiling water in an open vessel at a certain elevation, where the water goes from a liquid to. During an isobaric process, any heat added to the system will result in an increase in volume if the system does work on its surroundings. Boiling water is a classic example of an isobaric process. In the process, a gas either expands or contracts to maintain constant pressure, and hence the net amount of work is done by the system or on the system. What are are boiling of water and freezing of water categorized in with relevance to the 1st law of thermodynamics? When you heat water on the stove, you’re essentially. An example of the isobaric process includes the boiling of water to steam or the freezing of water to ice. Well, the vessel is open during the boiling of water.
from www.tec-science.com
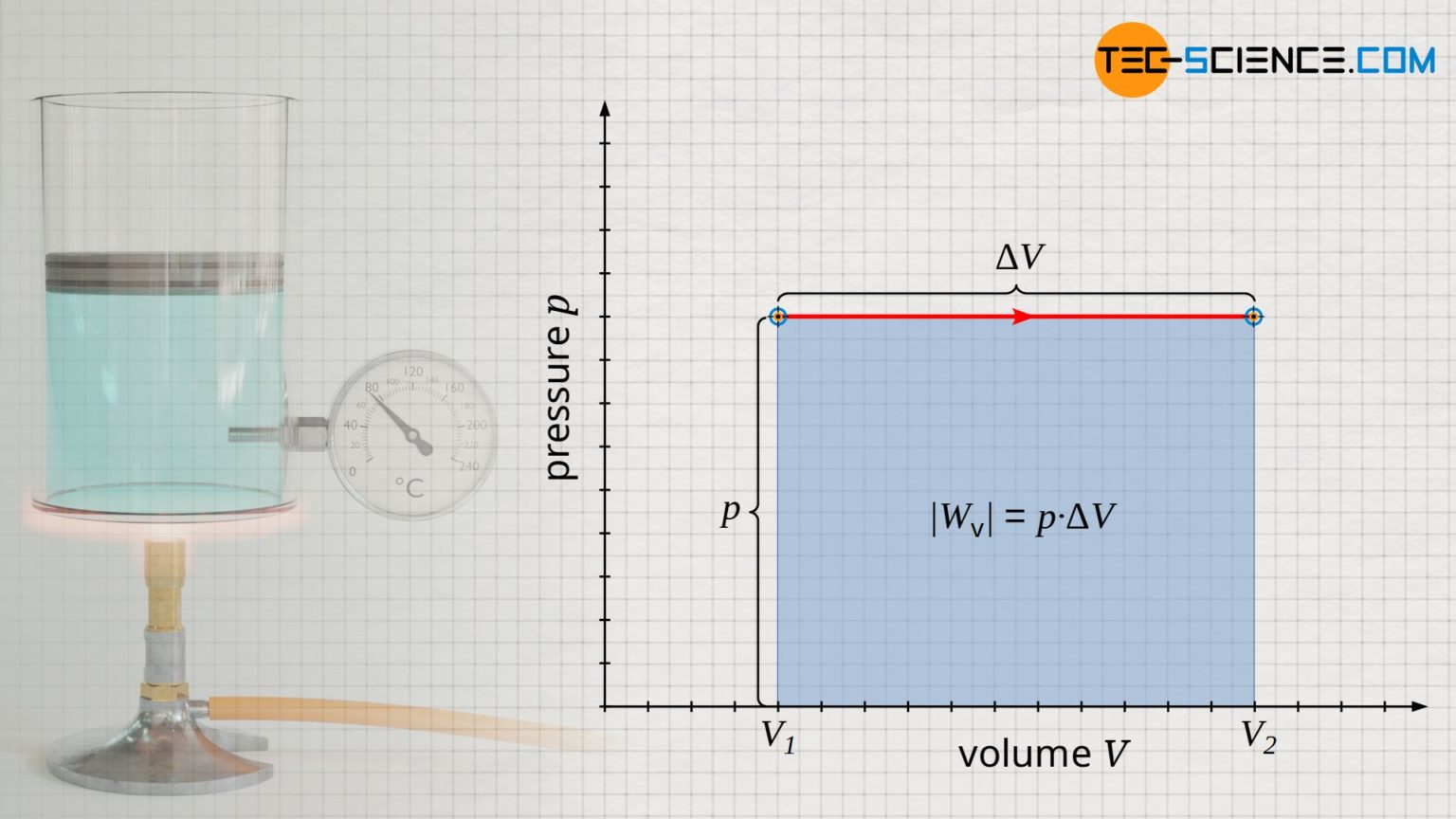
Well, the vessel is open during the boiling of water. Boiling water is a classic example of an isobaric process. When the volume of a system remains constant during a thermodynamic process, the process is called isochoric. An example of the isobaric process includes the boiling of water to steam or the freezing of water to ice. As the vessel is open, the pressure of the system will neither increase nor decrease. A quintessential example of an isobaric process is boiling water in an open vessel at a certain elevation, where the water goes from a liquid to. An isobaric process in action. What are are boiling of water and freezing of water categorized in with relevance to the 1st law of thermodynamics? During an isobaric process, any heat added to the system will result in an increase in volume if the system does work on its surroundings. When you heat water on the stove, you’re essentially.
Isobaric process in a closed system tecscience
Water Boiling Isobaric Process When the volume of a system remains constant during a thermodynamic process, the process is called isochoric. As the vessel is open, the pressure of the system will neither increase nor decrease. In the process, a gas either expands or contracts to maintain constant pressure, and hence the net amount of work is done by the system or on the system. Well, the vessel is open during the boiling of water. During an isobaric process, any heat added to the system will result in an increase in volume if the system does work on its surroundings. Boiling water is a classic example of an isobaric process. When the volume of a system remains constant during a thermodynamic process, the process is called isochoric. When you heat water on the stove, you’re essentially. A quintessential example of an isobaric process is boiling water in an open vessel at a certain elevation, where the water goes from a liquid to. An isobaric process in action. An example of the isobaric process includes the boiling of water to steam or the freezing of water to ice. What are are boiling of water and freezing of water categorized in with relevance to the 1st law of thermodynamics?
From www.etutorworld.com
Isobaric Process eTutorWorld Water Boiling Isobaric Process What are are boiling of water and freezing of water categorized in with relevance to the 1st law of thermodynamics? When the volume of a system remains constant during a thermodynamic process, the process is called isochoric. An isobaric process in action. An example of the isobaric process includes the boiling of water to steam or the freezing of water. Water Boiling Isobaric Process.
From www.youtube.com
Isobaric Process (Basics, pV diagram, Work Done, Change in Internal Energy & Enthalpy) Explained Water Boiling Isobaric Process As the vessel is open, the pressure of the system will neither increase nor decrease. During an isobaric process, any heat added to the system will result in an increase in volume if the system does work on its surroundings. When the volume of a system remains constant during a thermodynamic process, the process is called isochoric. What are are. Water Boiling Isobaric Process.
From www.researchgate.net
Vapor pressure of water and ice as well as isobaric mixing line with a... Download Scientific Water Boiling Isobaric Process During an isobaric process, any heat added to the system will result in an increase in volume if the system does work on its surroundings. In the process, a gas either expands or contracts to maintain constant pressure, and hence the net amount of work is done by the system or on the system. When the volume of a system. Water Boiling Isobaric Process.
From www.slideserve.com
PPT THERMODYNAMIC PowerPoint Presentation, free download ID6381537 Water Boiling Isobaric Process As the vessel is open, the pressure of the system will neither increase nor decrease. Well, the vessel is open during the boiling of water. An isobaric process in action. Boiling water is a classic example of an isobaric process. An example of the isobaric process includes the boiling of water to steam or the freezing of water to ice.. Water Boiling Isobaric Process.
From www.tec-science.com
Isobaric process in a closed system tecscience Water Boiling Isobaric Process Well, the vessel is open during the boiling of water. An example of the isobaric process includes the boiling of water to steam or the freezing of water to ice. As the vessel is open, the pressure of the system will neither increase nor decrease. When the volume of a system remains constant during a thermodynamic process, the process is. Water Boiling Isobaric Process.
From www.scribd.com
Isobaric Process Enthalpy Steam Water Boiling Isobaric Process Boiling water is a classic example of an isobaric process. When the volume of a system remains constant during a thermodynamic process, the process is called isochoric. An isobaric process in action. A quintessential example of an isobaric process is boiling water in an open vessel at a certain elevation, where the water goes from a liquid to. As the. Water Boiling Isobaric Process.
From www.youtube.com
Isobaric Process Thermodynamics Work Done by the Gas PV Diagram YouTube Water Boiling Isobaric Process An isobaric process in action. When the volume of a system remains constant during a thermodynamic process, the process is called isochoric. An example of the isobaric process includes the boiling of water to steam or the freezing of water to ice. What are are boiling of water and freezing of water categorized in with relevance to the 1st law. Water Boiling Isobaric Process.
From www.tec-science.com
Isobaric process in a closed system tecscience Water Boiling Isobaric Process In the process, a gas either expands or contracts to maintain constant pressure, and hence the net amount of work is done by the system or on the system. When the volume of a system remains constant during a thermodynamic process, the process is called isochoric. What are are boiling of water and freezing of water categorized in with relevance. Water Boiling Isobaric Process.
From journals.sagepub.com
Dynamic response characteristics of water and methane during isobaric imbibition process in Water Boiling Isobaric Process Well, the vessel is open during the boiling of water. A quintessential example of an isobaric process is boiling water in an open vessel at a certain elevation, where the water goes from a liquid to. Boiling water is a classic example of an isobaric process. An example of the isobaric process includes the boiling of water to steam or. Water Boiling Isobaric Process.
From www.researchgate.net
Density (solid black lines) and specific isobaric heat capacity (dashed... Download Scientific Water Boiling Isobaric Process A quintessential example of an isobaric process is boiling water in an open vessel at a certain elevation, where the water goes from a liquid to. Boiling water is a classic example of an isobaric process. Well, the vessel is open during the boiling of water. An isobaric process in action. When the volume of a system remains constant during. Water Boiling Isobaric Process.
From www.slideserve.com
PPT Laws of Thermodynamics PowerPoint Presentation, free download ID6592156 Water Boiling Isobaric Process As the vessel is open, the pressure of the system will neither increase nor decrease. What are are boiling of water and freezing of water categorized in with relevance to the 1st law of thermodynamics? An isobaric process in action. Well, the vessel is open during the boiling of water. An example of the isobaric process includes the boiling of. Water Boiling Isobaric Process.
From www.slideserve.com
PPT THERMODYNAMIC an introduction PowerPoint Presentation, free download ID2817183 Water Boiling Isobaric Process Boiling water is a classic example of an isobaric process. A quintessential example of an isobaric process is boiling water in an open vessel at a certain elevation, where the water goes from a liquid to. During an isobaric process, any heat added to the system will result in an increase in volume if the system does work on its. Water Boiling Isobaric Process.
From www.researchgate.net
Isobaric vapor−liquid equilibrium diagram for the IPA (1)−water... Download Scientific Diagram Water Boiling Isobaric Process Well, the vessel is open during the boiling of water. When the volume of a system remains constant during a thermodynamic process, the process is called isochoric. What are are boiling of water and freezing of water categorized in with relevance to the 1st law of thermodynamics? A quintessential example of an isobaric process is boiling water in an open. Water Boiling Isobaric Process.
From www.chemistrylearner.com
Physical Chemistry Chemistry Learner Water Boiling Isobaric Process An isobaric process in action. When the volume of a system remains constant during a thermodynamic process, the process is called isochoric. An example of the isobaric process includes the boiling of water to steam or the freezing of water to ice. Well, the vessel is open during the boiling of water. When you heat water on the stove, you’re. Water Boiling Isobaric Process.
From unacademy.com
Know In Detail About Isobaric Process And Its Importance Water Boiling Isobaric Process An example of the isobaric process includes the boiling of water to steam or the freezing of water to ice. During an isobaric process, any heat added to the system will result in an increase in volume if the system does work on its surroundings. When the volume of a system remains constant during a thermodynamic process, the process is. Water Boiling Isobaric Process.
From www.numerade.com
SOLVEDWater at 100 kPa, 25^∘ C is brought to the boiling point in a piston/cylinder with an Water Boiling Isobaric Process During an isobaric process, any heat added to the system will result in an increase in volume if the system does work on its surroundings. Boiling water is a classic example of an isobaric process. An isobaric process in action. In the process, a gas either expands or contracts to maintain constant pressure, and hence the net amount of work. Water Boiling Isobaric Process.
From www.slideshare.net
Chapter 15=Thermodynamics Water Boiling Isobaric Process As the vessel is open, the pressure of the system will neither increase nor decrease. Well, the vessel is open during the boiling of water. During an isobaric process, any heat added to the system will result in an increase in volume if the system does work on its surroundings. An example of the isobaric process includes the boiling of. Water Boiling Isobaric Process.
From www.researchgate.net
Schematics of the isobaric setup. Download Scientific Diagram Water Boiling Isobaric Process During an isobaric process, any heat added to the system will result in an increase in volume if the system does work on its surroundings. When you heat water on the stove, you’re essentially. Boiling water is a classic example of an isobaric process. A quintessential example of an isobaric process is boiling water in an open vessel at a. Water Boiling Isobaric Process.
From www.slideserve.com
PPT THERMODYNAMIC PowerPoint Presentation, free download ID9604221 Water Boiling Isobaric Process Well, the vessel is open during the boiling of water. What are are boiling of water and freezing of water categorized in with relevance to the 1st law of thermodynamics? Boiling water is a classic example of an isobaric process. During an isobaric process, any heat added to the system will result in an increase in volume if the system. Water Boiling Isobaric Process.
From www.etutorworld.com
Isobaric Process eTutorWorld Water Boiling Isobaric Process An example of the isobaric process includes the boiling of water to steam or the freezing of water to ice. A quintessential example of an isobaric process is boiling water in an open vessel at a certain elevation, where the water goes from a liquid to. During an isobaric process, any heat added to the system will result in an. Water Boiling Isobaric Process.
From www.tec-science.com
Isobaric process in a closed system tecscience Water Boiling Isobaric Process An example of the isobaric process includes the boiling of water to steam or the freezing of water to ice. When the volume of a system remains constant during a thermodynamic process, the process is called isochoric. An isobaric process in action. In the process, a gas either expands or contracts to maintain constant pressure, and hence the net amount. Water Boiling Isobaric Process.
From www.eigenplus.com
Fundamentals Of Isobaric Process Key Concepts, Work Done & Formulations eigenplus Water Boiling Isobaric Process An isobaric process in action. When the volume of a system remains constant during a thermodynamic process, the process is called isochoric. What are are boiling of water and freezing of water categorized in with relevance to the 1st law of thermodynamics? When you heat water on the stove, you’re essentially. As the vessel is open, the pressure of the. Water Boiling Isobaric Process.
From www.mecha-engineeringbd.com
Isobaric Process (Constant Pressure Process) Mechanical Engineering Water Boiling Isobaric Process Boiling water is a classic example of an isobaric process. When you heat water on the stove, you’re essentially. As the vessel is open, the pressure of the system will neither increase nor decrease. An example of the isobaric process includes the boiling of water to steam or the freezing of water to ice. Well, the vessel is open during. Water Boiling Isobaric Process.
From www.youtube.com
Thermodynamic Processes Isobaric, Isochoric, Isothermal and Adiabatic process Chemistry 12 Water Boiling Isobaric Process As the vessel is open, the pressure of the system will neither increase nor decrease. Boiling water is a classic example of an isobaric process. What are are boiling of water and freezing of water categorized in with relevance to the 1st law of thermodynamics? Well, the vessel is open during the boiling of water. During an isobaric process, any. Water Boiling Isobaric Process.
From www.vecteezy.com
Boiling Water Process Vector Flat Illustration 161668 Vector Art at Vecteezy Water Boiling Isobaric Process Boiling water is a classic example of an isobaric process. Well, the vessel is open during the boiling of water. An isobaric process in action. In the process, a gas either expands or contracts to maintain constant pressure, and hence the net amount of work is done by the system or on the system. When you heat water on the. Water Boiling Isobaric Process.
From www.slideserve.com
PPT Do Now PowerPoint Presentation, free download ID6329644 Water Boiling Isobaric Process An example of the isobaric process includes the boiling of water to steam or the freezing of water to ice. Well, the vessel is open during the boiling of water. A quintessential example of an isobaric process is boiling water in an open vessel at a certain elevation, where the water goes from a liquid to. An isobaric process in. Water Boiling Isobaric Process.
From www.eigenplus.com
Fundamentals Of Isobaric Process Key Concepts, Work Done & Formulations eigenplus Water Boiling Isobaric Process An isobaric process in action. Well, the vessel is open during the boiling of water. When you heat water on the stove, you’re essentially. During an isobaric process, any heat added to the system will result in an increase in volume if the system does work on its surroundings. Boiling water is a classic example of an isobaric process. When. Water Boiling Isobaric Process.
From www.slideserve.com
PPT Thermodynamics PowerPoint Presentation, free download ID6778399 Water Boiling Isobaric Process When you heat water on the stove, you’re essentially. What are are boiling of water and freezing of water categorized in with relevance to the 1st law of thermodynamics? Boiling water is a classic example of an isobaric process. During an isobaric process, any heat added to the system will result in an increase in volume if the system does. Water Boiling Isobaric Process.
From www.etutorworld.com
Isobaric Process eTutorWorld Water Boiling Isobaric Process Boiling water is a classic example of an isobaric process. As the vessel is open, the pressure of the system will neither increase nor decrease. During an isobaric process, any heat added to the system will result in an increase in volume if the system does work on its surroundings. An example of the isobaric process includes the boiling of. Water Boiling Isobaric Process.
From www.chegg.com
Solved FIGURE P6.57 6.58 Water at 100 kPa, 25°C is brought Water Boiling Isobaric Process Boiling water is a classic example of an isobaric process. As the vessel is open, the pressure of the system will neither increase nor decrease. When the volume of a system remains constant during a thermodynamic process, the process is called isochoric. When you heat water on the stove, you’re essentially. A quintessential example of an isobaric process is boiling. Water Boiling Isobaric Process.
From www.youtube.com
Isobaric Process Thermodynamics Work & Heat Energy, Molar Heat Capacity, & Internal Energy Water Boiling Isobaric Process As the vessel is open, the pressure of the system will neither increase nor decrease. When the volume of a system remains constant during a thermodynamic process, the process is called isochoric. In the process, a gas either expands or contracts to maintain constant pressure, and hence the net amount of work is done by the system or on the. Water Boiling Isobaric Process.
From lawofthermodynamicsinfo.com
Thermodynamic Process (With Examples) Isobaric, Isochoric, Adiabatic, Isothermal Water Boiling Isobaric Process As the vessel is open, the pressure of the system will neither increase nor decrease. During an isobaric process, any heat added to the system will result in an increase in volume if the system does work on its surroundings. Well, the vessel is open during the boiling of water. When the volume of a system remains constant during a. Water Boiling Isobaric Process.
From www.slideserve.com
PPT Work in Thermodynamic Processes PowerPoint Presentation, free download ID6973705 Water Boiling Isobaric Process During an isobaric process, any heat added to the system will result in an increase in volume if the system does work on its surroundings. As the vessel is open, the pressure of the system will neither increase nor decrease. When you heat water on the stove, you’re essentially. When the volume of a system remains constant during a thermodynamic. Water Boiling Isobaric Process.
From en.wikipedia.org
Isobaric process Wikipedia Water Boiling Isobaric Process An example of the isobaric process includes the boiling of water to steam or the freezing of water to ice. Boiling water is a classic example of an isobaric process. When you heat water on the stove, you’re essentially. Well, the vessel is open during the boiling of water. In the process, a gas either expands or contracts to maintain. Water Boiling Isobaric Process.
From www.researchgate.net
Heat Engine with Isobaric Process. Top left figure shows the initial... Download Scientific Water Boiling Isobaric Process An example of the isobaric process includes the boiling of water to steam or the freezing of water to ice. What are are boiling of water and freezing of water categorized in with relevance to the 1st law of thermodynamics? Boiling water is a classic example of an isobaric process. In the process, a gas either expands or contracts to. Water Boiling Isobaric Process.