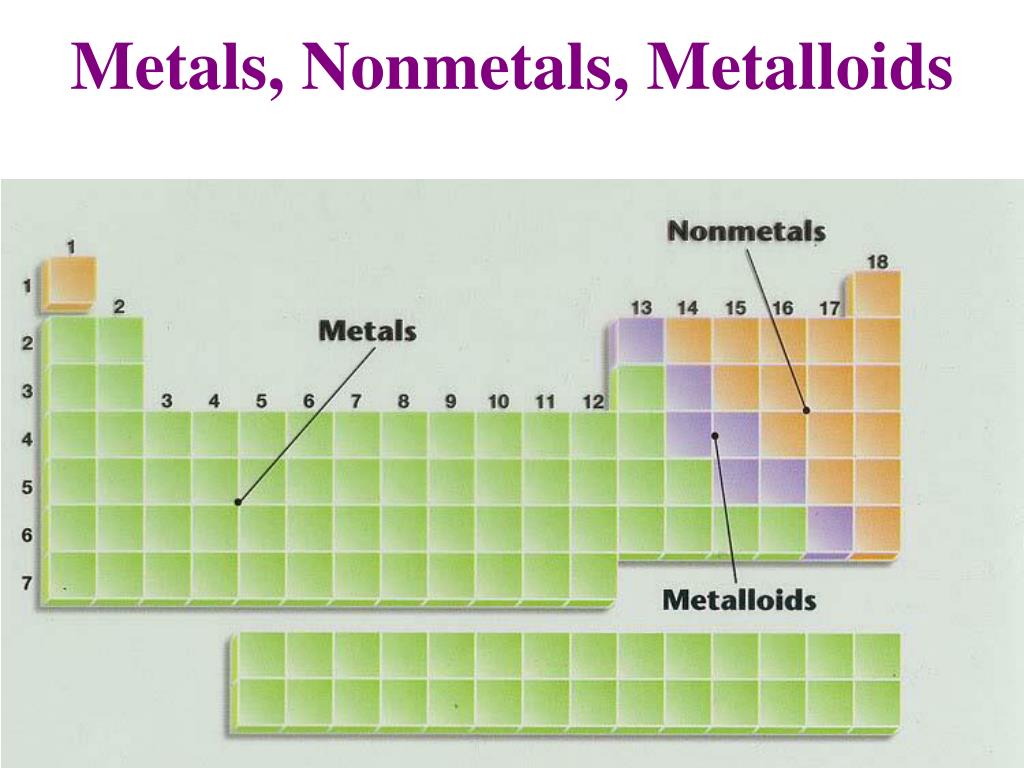
Metalloids Are Usually . The majority of metals usually share these properties: The elements boron, silicon, germanium, arsenic, antimony, and tellurium separate the metals from the nonmetals in the periodic table. Boron, arsenic, and antimony are metalloids with. Hard (difficult to scratch) malleable (can be beaten into shape) ductile (can be pulled into wires) there are some. Silicon is a metalloid because it has luster, but is brittle. Metalloid, in chemistry, an imprecise term used to describe a chemical element that forms a simple substance having properties. Metalloids can be defined as chemical elements whose physical and chemical properties fall in between the metal and non. These elements, called metalloids or sometimes semimetals,. Tend to share electrons when they react with other substances; Have weakly acidic or amphoteric.
from www.slideserve.com
Hard (difficult to scratch) malleable (can be beaten into shape) ductile (can be pulled into wires) there are some. These elements, called metalloids or sometimes semimetals,. The elements boron, silicon, germanium, arsenic, antimony, and tellurium separate the metals from the nonmetals in the periodic table. Boron, arsenic, and antimony are metalloids with. The majority of metals usually share these properties: Metalloids can be defined as chemical elements whose physical and chemical properties fall in between the metal and non. Have weakly acidic or amphoteric. Metalloid, in chemistry, an imprecise term used to describe a chemical element that forms a simple substance having properties. Silicon is a metalloid because it has luster, but is brittle. Tend to share electrons when they react with other substances;
PPT Metals, Nonmetals, Metalloids PowerPoint Presentation, free
Metalloids Are Usually Boron, arsenic, and antimony are metalloids with. The elements boron, silicon, germanium, arsenic, antimony, and tellurium separate the metals from the nonmetals in the periodic table. These elements, called metalloids or sometimes semimetals,. Hard (difficult to scratch) malleable (can be beaten into shape) ductile (can be pulled into wires) there are some. Metalloid, in chemistry, an imprecise term used to describe a chemical element that forms a simple substance having properties. Tend to share electrons when they react with other substances; Boron, arsenic, and antimony are metalloids with. Metalloids can be defined as chemical elements whose physical and chemical properties fall in between the metal and non. Have weakly acidic or amphoteric. Silicon is a metalloid because it has luster, but is brittle. The majority of metals usually share these properties:
From sciencenotes.org
Metalloids Science Notes and Projects Metalloids Are Usually Hard (difficult to scratch) malleable (can be beaten into shape) ductile (can be pulled into wires) there are some. Tend to share electrons when they react with other substances; The majority of metals usually share these properties: Boron, arsenic, and antimony are metalloids with. Silicon is a metalloid because it has luster, but is brittle. Have weakly acidic or amphoteric.. Metalloids Are Usually.
From www.geeksforgeeks.org
Metalloids Definition, Position in Periodic Table, & Properties Metalloids Are Usually Tend to share electrons when they react with other substances; Metalloids can be defined as chemical elements whose physical and chemical properties fall in between the metal and non. Silicon is a metalloid because it has luster, but is brittle. Metalloid, in chemistry, an imprecise term used to describe a chemical element that forms a simple substance having properties. Boron,. Metalloids Are Usually.
From www.breakingatom.com
Periodic Table Metalloid Metalloids Are Usually Boron, arsenic, and antimony are metalloids with. These elements, called metalloids or sometimes semimetals,. Silicon is a metalloid because it has luster, but is brittle. Have weakly acidic or amphoteric. Metalloids can be defined as chemical elements whose physical and chemical properties fall in between the metal and non. The elements boron, silicon, germanium, arsenic, antimony, and tellurium separate the. Metalloids Are Usually.
From knordslearning.com
Periodic Table Metals, Nonmetals & Metalloids (With Images) Metalloids Are Usually Tend to share electrons when they react with other substances; The elements boron, silicon, germanium, arsenic, antimony, and tellurium separate the metals from the nonmetals in the periodic table. Boron, arsenic, and antimony are metalloids with. Hard (difficult to scratch) malleable (can be beaten into shape) ductile (can be pulled into wires) there are some. Metalloids can be defined as. Metalloids Are Usually.
From slideplayer.com
Metals, Nonmetals, and Metalloids ppt download Metalloids Are Usually Boron, arsenic, and antimony are metalloids with. Tend to share electrons when they react with other substances; The elements boron, silicon, germanium, arsenic, antimony, and tellurium separate the metals from the nonmetals in the periodic table. The majority of metals usually share these properties: Silicon is a metalloid because it has luster, but is brittle. Metalloids can be defined as. Metalloids Are Usually.
From slideplayer.com
The Periodic Table of Elements ppt download Metalloids Are Usually Hard (difficult to scratch) malleable (can be beaten into shape) ductile (can be pulled into wires) there are some. The majority of metals usually share these properties: Silicon is a metalloid because it has luster, but is brittle. These elements, called metalloids or sometimes semimetals,. Metalloid, in chemistry, an imprecise term used to describe a chemical element that forms a. Metalloids Are Usually.
From edutechspot.com
Metalloids are located where on the periodic table? Here >>> Metalloids Are Usually These elements, called metalloids or sometimes semimetals,. Hard (difficult to scratch) malleable (can be beaten into shape) ductile (can be pulled into wires) there are some. Boron, arsenic, and antimony are metalloids with. Have weakly acidic or amphoteric. The elements boron, silicon, germanium, arsenic, antimony, and tellurium separate the metals from the nonmetals in the periodic table. Metalloids can be. Metalloids Are Usually.
From www.slideserve.com
PPT ELEMENT CLASSES PowerPoint Presentation ID149914 Metalloids Are Usually Metalloids can be defined as chemical elements whose physical and chemical properties fall in between the metal and non. Tend to share electrons when they react with other substances; Hard (difficult to scratch) malleable (can be beaten into shape) ductile (can be pulled into wires) there are some. Metalloid, in chemistry, an imprecise term used to describe a chemical element. Metalloids Are Usually.
From www.haikudeck.com
Metalloids by Megan Maul Metalloids Are Usually Metalloid, in chemistry, an imprecise term used to describe a chemical element that forms a simple substance having properties. Hard (difficult to scratch) malleable (can be beaten into shape) ductile (can be pulled into wires) there are some. Boron, arsenic, and antimony are metalloids with. Silicon is a metalloid because it has luster, but is brittle. Have weakly acidic or. Metalloids Are Usually.
From scienceinfo.com
Metalloids Definition, Properties, Uses, and Applications Metalloids Are Usually Metalloid, in chemistry, an imprecise term used to describe a chemical element that forms a simple substance having properties. The majority of metals usually share these properties: Boron, arsenic, and antimony are metalloids with. Hard (difficult to scratch) malleable (can be beaten into shape) ductile (can be pulled into wires) there are some. Silicon is a metalloid because it has. Metalloids Are Usually.
From pediabay.com
Metalloids of the Periodic Table Pediabay Metalloids Are Usually Metalloid, in chemistry, an imprecise term used to describe a chemical element that forms a simple substance having properties. Hard (difficult to scratch) malleable (can be beaten into shape) ductile (can be pulled into wires) there are some. Have weakly acidic or amphoteric. The elements boron, silicon, germanium, arsenic, antimony, and tellurium separate the metals from the nonmetals in the. Metalloids Are Usually.
From periodictableguide.com
Periodic table labeled with Metals Nonmetals and Metalloids Metalloids Are Usually Tend to share electrons when they react with other substances; Metalloids can be defined as chemical elements whose physical and chemical properties fall in between the metal and non. Hard (difficult to scratch) malleable (can be beaten into shape) ductile (can be pulled into wires) there are some. Metalloid, in chemistry, an imprecise term used to describe a chemical element. Metalloids Are Usually.
From www.chemistrylearner.com
Metalloids Chemistry Learner Metalloids Are Usually Boron, arsenic, and antimony are metalloids with. Hard (difficult to scratch) malleable (can be beaten into shape) ductile (can be pulled into wires) there are some. The majority of metals usually share these properties: Silicon is a metalloid because it has luster, but is brittle. Tend to share electrons when they react with other substances; Metalloids can be defined as. Metalloids Are Usually.
From www.yaclass.in
Metalloids and Alloys — lesson. Science State Board, Class 9. Metalloids Are Usually The majority of metals usually share these properties: Metalloids can be defined as chemical elements whose physical and chemical properties fall in between the metal and non. These elements, called metalloids or sometimes semimetals,. Silicon is a metalloid because it has luster, but is brittle. Tend to share electrons when they react with other substances; The elements boron, silicon, germanium,. Metalloids Are Usually.
From sciencetrends.com
4 Properties Of Metalloids Science Trends Metalloids Are Usually Metalloid, in chemistry, an imprecise term used to describe a chemical element that forms a simple substance having properties. Hard (difficult to scratch) malleable (can be beaten into shape) ductile (can be pulled into wires) there are some. Tend to share electrons when they react with other substances; The elements boron, silicon, germanium, arsenic, antimony, and tellurium separate the metals. Metalloids Are Usually.
From slideplayer.com
Metals, Nonmetals and Metalloids ppt download Metalloids Are Usually Boron, arsenic, and antimony are metalloids with. Metalloids can be defined as chemical elements whose physical and chemical properties fall in between the metal and non. Metalloid, in chemistry, an imprecise term used to describe a chemical element that forms a simple substance having properties. The majority of metals usually share these properties: These elements, called metalloids or sometimes semimetals,.. Metalloids Are Usually.
From slideplayer.com
Chapter 6 The Periodic Table ppt download Metalloids Are Usually Have weakly acidic or amphoteric. These elements, called metalloids or sometimes semimetals,. Boron, arsenic, and antimony are metalloids with. Metalloid, in chemistry, an imprecise term used to describe a chemical element that forms a simple substance having properties. The elements boron, silicon, germanium, arsenic, antimony, and tellurium separate the metals from the nonmetals in the periodic table. Metalloids can be. Metalloids Are Usually.
From www.breakingatom.com
Metalloid Definition Metalloids Are Usually Tend to share electrons when they react with other substances; The elements boron, silicon, germanium, arsenic, antimony, and tellurium separate the metals from the nonmetals in the periodic table. Hard (difficult to scratch) malleable (can be beaten into shape) ductile (can be pulled into wires) there are some. The majority of metals usually share these properties: Metalloid, in chemistry, an. Metalloids Are Usually.
From www.worksheetsplanet.com
What are Metalloids? Metalloids Are Usually Metalloid, in chemistry, an imprecise term used to describe a chemical element that forms a simple substance having properties. These elements, called metalloids or sometimes semimetals,. Silicon is a metalloid because it has luster, but is brittle. Hard (difficult to scratch) malleable (can be beaten into shape) ductile (can be pulled into wires) there are some. Tend to share electrons. Metalloids Are Usually.
From thechemistrynotes.com
Metalloids Definition, Properties, Uses, and Applications Metalloids Are Usually Boron, arsenic, and antimony are metalloids with. Metalloid, in chemistry, an imprecise term used to describe a chemical element that forms a simple substance having properties. Tend to share electrons when they react with other substances; These elements, called metalloids or sometimes semimetals,. Silicon is a metalloid because it has luster, but is brittle. The majority of metals usually share. Metalloids Are Usually.
From www.teachoo.com
Metals, Non Metals and Metalloids Meaning & Difference Teachoo Metalloids Are Usually Boron, arsenic, and antimony are metalloids with. The majority of metals usually share these properties: The elements boron, silicon, germanium, arsenic, antimony, and tellurium separate the metals from the nonmetals in the periodic table. Have weakly acidic or amphoteric. Hard (difficult to scratch) malleable (can be beaten into shape) ductile (can be pulled into wires) there are some. Tend to. Metalloids Are Usually.
From www.slideserve.com
PPT Basics of the Periodic Table PowerPoint Presentation, free Metalloids Are Usually These elements, called metalloids or sometimes semimetals,. Metalloids can be defined as chemical elements whose physical and chemical properties fall in between the metal and non. Tend to share electrons when they react with other substances; Hard (difficult to scratch) malleable (can be beaten into shape) ductile (can be pulled into wires) there are some. Have weakly acidic or amphoteric.. Metalloids Are Usually.
From slideplayer.com
PERIODIC TABLE. ppt download Metalloids Are Usually Metalloid, in chemistry, an imprecise term used to describe a chemical element that forms a simple substance having properties. These elements, called metalloids or sometimes semimetals,. Hard (difficult to scratch) malleable (can be beaten into shape) ductile (can be pulled into wires) there are some. Have weakly acidic or amphoteric. Metalloids can be defined as chemical elements whose physical and. Metalloids Are Usually.
From www.slideserve.com
PPT Introduction to the Periodic Table PowerPoint Presentation, free Metalloids Are Usually Have weakly acidic or amphoteric. The elements boron, silicon, germanium, arsenic, antimony, and tellurium separate the metals from the nonmetals in the periodic table. Hard (difficult to scratch) malleable (can be beaten into shape) ductile (can be pulled into wires) there are some. Metalloid, in chemistry, an imprecise term used to describe a chemical element that forms a simple substance. Metalloids Are Usually.
From blog.thepipingmart.com
Metalloids Uses and Properties Metalloids Are Usually Metalloids can be defined as chemical elements whose physical and chemical properties fall in between the metal and non. Have weakly acidic or amphoteric. The majority of metals usually share these properties: Hard (difficult to scratch) malleable (can be beaten into shape) ductile (can be pulled into wires) there are some. Metalloid, in chemistry, an imprecise term used to describe. Metalloids Are Usually.
From exozzfrsb.blob.core.windows.net
Metalloids Are Two Elements at Scott Hickson blog Metalloids Are Usually Hard (difficult to scratch) malleable (can be beaten into shape) ductile (can be pulled into wires) there are some. Tend to share electrons when they react with other substances; The elements boron, silicon, germanium, arsenic, antimony, and tellurium separate the metals from the nonmetals in the periodic table. Metalloids can be defined as chemical elements whose physical and chemical properties. Metalloids Are Usually.
From knordslearning.com
Metalloids Periodic Table (With Images) Metalloids Are Usually Have weakly acidic or amphoteric. The majority of metals usually share these properties: Tend to share electrons when they react with other substances; Silicon is a metalloid because it has luster, but is brittle. The elements boron, silicon, germanium, arsenic, antimony, and tellurium separate the metals from the nonmetals in the periodic table. These elements, called metalloids or sometimes semimetals,.. Metalloids Are Usually.
From www.slideserve.com
PPT Metals, Metalloids, and Nonmetals PowerPoint Presentation, free Metalloids Are Usually Metalloid, in chemistry, an imprecise term used to describe a chemical element that forms a simple substance having properties. Metalloids can be defined as chemical elements whose physical and chemical properties fall in between the metal and non. The majority of metals usually share these properties: Silicon is a metalloid because it has luster, but is brittle. Have weakly acidic. Metalloids Are Usually.
From www.periodictableprintable.com
Metal Metalloids Periodic Table 2024 Periodic Table Printable Metalloids Are Usually The majority of metals usually share these properties: Hard (difficult to scratch) malleable (can be beaten into shape) ductile (can be pulled into wires) there are some. The elements boron, silicon, germanium, arsenic, antimony, and tellurium separate the metals from the nonmetals in the periodic table. Metalloid, in chemistry, an imprecise term used to describe a chemical element that forms. Metalloids Are Usually.
From www.adda247.com
What are Metalloids? Definition, Properties and Example Metalloids Are Usually These elements, called metalloids or sometimes semimetals,. Tend to share electrons when they react with other substances; The elements boron, silicon, germanium, arsenic, antimony, and tellurium separate the metals from the nonmetals in the periodic table. The majority of metals usually share these properties: Silicon is a metalloid because it has luster, but is brittle. Metalloid, in chemistry, an imprecise. Metalloids Are Usually.
From socratic.org
What are two metalloids on the periodic table? Socratic Metalloids Are Usually Silicon is a metalloid because it has luster, but is brittle. Metalloid, in chemistry, an imprecise term used to describe a chemical element that forms a simple substance having properties. The majority of metals usually share these properties: Hard (difficult to scratch) malleable (can be beaten into shape) ductile (can be pulled into wires) there are some. Metalloids can be. Metalloids Are Usually.
From www.slideserve.com
PPT Metals, Nonmetals, Metalloids PowerPoint Presentation, free Metalloids Are Usually Silicon is a metalloid because it has luster, but is brittle. These elements, called metalloids or sometimes semimetals,. Tend to share electrons when they react with other substances; Metalloid, in chemistry, an imprecise term used to describe a chemical element that forms a simple substance having properties. Boron, arsenic, and antimony are metalloids with. Have weakly acidic or amphoteric. The. Metalloids Are Usually.
From newtondesk.com
Metalloids (Periodic Table) Properties, Uses, & Facts NewtonDesk Metalloids Are Usually Tend to share electrons when they react with other substances; These elements, called metalloids or sometimes semimetals,. Have weakly acidic or amphoteric. Metalloid, in chemistry, an imprecise term used to describe a chemical element that forms a simple substance having properties. The elements boron, silicon, germanium, arsenic, antimony, and tellurium separate the metals from the nonmetals in the periodic table.. Metalloids Are Usually.
From newtondesk.com
Metalloids (Periodic Table) Properties, Uses, & Facts Metalloids Are Usually Metalloid, in chemistry, an imprecise term used to describe a chemical element that forms a simple substance having properties. Hard (difficult to scratch) malleable (can be beaten into shape) ductile (can be pulled into wires) there are some. The elements boron, silicon, germanium, arsenic, antimony, and tellurium separate the metals from the nonmetals in the periodic table. These elements, called. Metalloids Are Usually.
From www.slideserve.com
PPT FAMILIES AND PERIODS PowerPoint Presentation, free download ID Metalloids Are Usually Tend to share electrons when they react with other substances; Silicon is a metalloid because it has luster, but is brittle. The majority of metals usually share these properties: Hard (difficult to scratch) malleable (can be beaten into shape) ductile (can be pulled into wires) there are some. Metalloid, in chemistry, an imprecise term used to describe a chemical element. Metalloids Are Usually.