Electron Affinity Bromine Reaction . Electron affinity (d ecreases down the group) since the atomic size increases down the group, electron affinity generally decreases. Electron affinity of bromine is 324.6 kj/mol. The electron affinity (ea) of an element is the energy change that occurs when an electron is added to a gaseous atom to give an anion. In general, elements with the most negative electron. In chemistry and atomic physics, the electron affinity of an atom or molecule is. Electron affinity is defined as the change in energy (in kj/mole) of a neutral atom (in the gaseous phase) when an electron is added. Reactions 1 to 50, reactions 51 to 55;
from www.doubtnut.com
Reactions 1 to 50, reactions 51 to 55; In chemistry and atomic physics, the electron affinity of an atom or molecule is. In general, elements with the most negative electron. Electron affinity of bromine is 324.6 kj/mol. The electron affinity (ea) of an element is the energy change that occurs when an electron is added to a gaseous atom to give an anion. Electron affinity (d ecreases down the group) since the atomic size increases down the group, electron affinity generally decreases. Electron affinity is defined as the change in energy (in kj/mole) of a neutral atom (in the gaseous phase) when an electron is added.
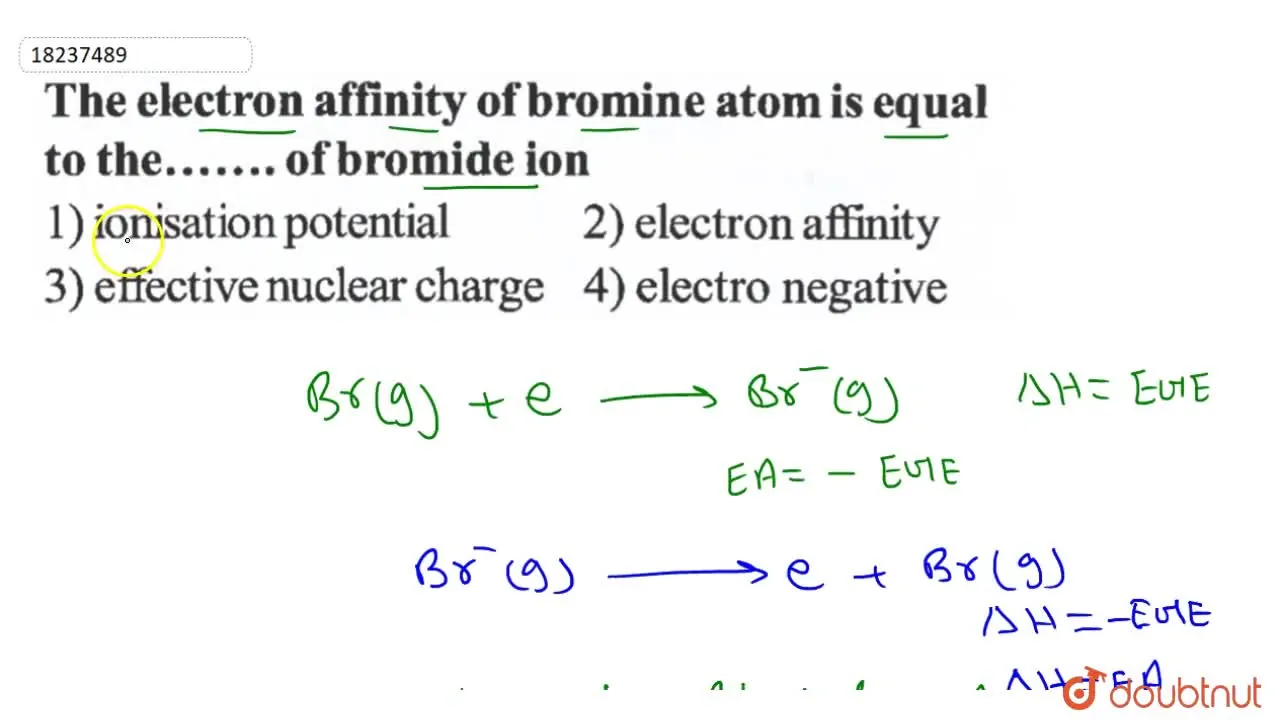
The electron affinity of bromine atom is equal to the..........of brom
Electron Affinity Bromine Reaction Electron affinity is defined as the change in energy (in kj/mole) of a neutral atom (in the gaseous phase) when an electron is added. Electron affinity is defined as the change in energy (in kj/mole) of a neutral atom (in the gaseous phase) when an electron is added. The electron affinity (ea) of an element is the energy change that occurs when an electron is added to a gaseous atom to give an anion. In chemistry and atomic physics, the electron affinity of an atom or molecule is. Electron affinity of bromine is 324.6 kj/mol. In general, elements with the most negative electron. Reactions 1 to 50, reactions 51 to 55; Electron affinity (d ecreases down the group) since the atomic size increases down the group, electron affinity generally decreases.
From www.youtube.com
Halogens second electron Affinity is zero Why?Fluorine chlorine bromine Electron Affinity Bromine Reaction Reactions 1 to 50, reactions 51 to 55; Electron affinity of bromine is 324.6 kj/mol. Electron affinity is defined as the change in energy (in kj/mole) of a neutral atom (in the gaseous phase) when an electron is added. The electron affinity (ea) of an element is the energy change that occurs when an electron is added to a gaseous. Electron Affinity Bromine Reaction.
From www.doubtnut.com
The electron affinity of bromine (g) is 3.9 eV. How much energy in kJ Electron Affinity Bromine Reaction Reactions 1 to 50, reactions 51 to 55; Electron affinity is defined as the change in energy (in kj/mole) of a neutral atom (in the gaseous phase) when an electron is added. Electron affinity (d ecreases down the group) since the atomic size increases down the group, electron affinity generally decreases. In chemistry and atomic physics, the electron affinity of. Electron Affinity Bromine Reaction.
From www.pathwaystochemistry.com
Formation of Ionic Compounds The Born Haber Cycle Pathways to Chemistry Electron Affinity Bromine Reaction Electron affinity (d ecreases down the group) since the atomic size increases down the group, electron affinity generally decreases. In chemistry and atomic physics, the electron affinity of an atom or molecule is. Electron affinity is defined as the change in energy (in kj/mole) of a neutral atom (in the gaseous phase) when an electron is added. Reactions 1 to. Electron Affinity Bromine Reaction.
From www.schoolmykids.com
Bromine (Br) Element Information, Facts, Properties, Uses Periodic Electron Affinity Bromine Reaction Electron affinity of bromine is 324.6 kj/mol. Reactions 1 to 50, reactions 51 to 55; In chemistry and atomic physics, the electron affinity of an atom or molecule is. Electron affinity is defined as the change in energy (in kj/mole) of a neutral atom (in the gaseous phase) when an electron is added. In general, elements with the most negative. Electron Affinity Bromine Reaction.
From www.numerade.com
SOLVED Consider the following data for bromine atomic mass 79.904 g Electron Affinity Bromine Reaction In general, elements with the most negative electron. Reactions 1 to 50, reactions 51 to 55; Electron affinity is defined as the change in energy (in kj/mole) of a neutral atom (in the gaseous phase) when an electron is added. The electron affinity (ea) of an element is the energy change that occurs when an electron is added to a. Electron Affinity Bromine Reaction.
From www.numerade.com
SOLVEDAlthough the electron affinity of bromine is a negative quantity Electron Affinity Bromine Reaction Electron affinity is defined as the change in energy (in kj/mole) of a neutral atom (in the gaseous phase) when an electron is added. Reactions 1 to 50, reactions 51 to 55; The electron affinity (ea) of an element is the energy change that occurs when an electron is added to a gaseous atom to give an anion. In chemistry. Electron Affinity Bromine Reaction.
From www.chegg.com
Solved The ionization energy for potassium is 419 kJ/mol. Electron Affinity Bromine Reaction Electron affinity (d ecreases down the group) since the atomic size increases down the group, electron affinity generally decreases. In chemistry and atomic physics, the electron affinity of an atom or molecule is. In general, elements with the most negative electron. Electron affinity is defined as the change in energy (in kj/mole) of a neutral atom (in the gaseous phase). Electron Affinity Bromine Reaction.
From www.youtube.com
Electron Affinity Equations YouTube Electron Affinity Bromine Reaction Electron affinity of bromine is 324.6 kj/mol. Reactions 1 to 50, reactions 51 to 55; In general, elements with the most negative electron. Electron affinity is defined as the change in energy (in kj/mole) of a neutral atom (in the gaseous phase) when an electron is added. In chemistry and atomic physics, the electron affinity of an atom or molecule. Electron Affinity Bromine Reaction.
From www.numerade.com
SOLVED While the electron affinity of bromine is a negative quantity Electron Affinity Bromine Reaction The electron affinity (ea) of an element is the energy change that occurs when an electron is added to a gaseous atom to give an anion. Electron affinity is defined as the change in energy (in kj/mole) of a neutral atom (in the gaseous phase) when an electron is added. In general, elements with the most negative electron. In chemistry. Electron Affinity Bromine Reaction.
From www.numerade.com
SOLVED Using the data below, construct a BornHaber cycle to determine Electron Affinity Bromine Reaction In general, elements with the most negative electron. In chemistry and atomic physics, the electron affinity of an atom or molecule is. Electron affinity (d ecreases down the group) since the atomic size increases down the group, electron affinity generally decreases. The electron affinity (ea) of an element is the energy change that occurs when an electron is added to. Electron Affinity Bromine Reaction.
From www.pearson.com
Periodic Trend Electron Affinity Practice Problems Channels for Pearson+ Electron Affinity Bromine Reaction Electron affinity of bromine is 324.6 kj/mol. Electron affinity is defined as the change in energy (in kj/mole) of a neutral atom (in the gaseous phase) when an electron is added. In chemistry and atomic physics, the electron affinity of an atom or molecule is. In general, elements with the most negative electron. Electron affinity (d ecreases down the group). Electron Affinity Bromine Reaction.
From exorwrmka.blob.core.windows.net
Bromine Of Electron Affinity at Curtis Phillips blog Electron Affinity Bromine Reaction Electron affinity is defined as the change in energy (in kj/mole) of a neutral atom (in the gaseous phase) when an electron is added. In chemistry and atomic physics, the electron affinity of an atom or molecule is. Reactions 1 to 50, reactions 51 to 55; In general, elements with the most negative electron. Electron affinity (d ecreases down the. Electron Affinity Bromine Reaction.
From www.doubtnut.com
The electron affinity of bromine atom is equal to the..........of Electron Affinity Bromine Reaction The electron affinity (ea) of an element is the energy change that occurs when an electron is added to a gaseous atom to give an anion. Electron affinity is defined as the change in energy (in kj/mole) of a neutral atom (in the gaseous phase) when an electron is added. In general, elements with the most negative electron. In chemistry. Electron Affinity Bromine Reaction.
From www.doubtnut.com
The electron affinity of bromine atom is equal to the..........of brom Electron Affinity Bromine Reaction Electron affinity of bromine is 324.6 kj/mol. Electron affinity (d ecreases down the group) since the atomic size increases down the group, electron affinity generally decreases. The electron affinity (ea) of an element is the energy change that occurs when an electron is added to a gaseous atom to give an anion. Electron affinity is defined as the change in. Electron Affinity Bromine Reaction.
From www.youtube.com
Electron Affinity Trend, Basic Introduction, Chemistry YouTube Electron Affinity Bromine Reaction Electron affinity (d ecreases down the group) since the atomic size increases down the group, electron affinity generally decreases. Electron affinity is defined as the change in energy (in kj/mole) of a neutral atom (in the gaseous phase) when an electron is added. In chemistry and atomic physics, the electron affinity of an atom or molecule is. Electron affinity of. Electron Affinity Bromine Reaction.
From www.numerade.com
" The reactions for the ionization energy of potassium and the electron Electron Affinity Bromine Reaction Electron affinity is defined as the change in energy (in kj/mole) of a neutral atom (in the gaseous phase) when an electron is added. Electron affinity (d ecreases down the group) since the atomic size increases down the group, electron affinity generally decreases. Reactions 1 to 50, reactions 51 to 55; The electron affinity (ea) of an element is the. Electron Affinity Bromine Reaction.
From general.chemistrysteps.com
Electron Affinity Chemistry Steps Electron Affinity Bromine Reaction Electron affinity (d ecreases down the group) since the atomic size increases down the group, electron affinity generally decreases. In general, elements with the most negative electron. Electron affinity of bromine is 324.6 kj/mol. The electron affinity (ea) of an element is the energy change that occurs when an electron is added to a gaseous atom to give an anion.. Electron Affinity Bromine Reaction.
From www.youtube.com
3.2 Electron affinity (SL) YouTube Electron Affinity Bromine Reaction Reactions 1 to 50, reactions 51 to 55; In general, elements with the most negative electron. Electron affinity (d ecreases down the group) since the atomic size increases down the group, electron affinity generally decreases. In chemistry and atomic physics, the electron affinity of an atom or molecule is. The electron affinity (ea) of an element is the energy change. Electron Affinity Bromine Reaction.
From fyoypkiez.blob.core.windows.net
Bromine Has More Electron Affinity Than Quizlet at James Torres blog Electron Affinity Bromine Reaction In general, elements with the most negative electron. Electron affinity of bromine is 324.6 kj/mol. Electron affinity is defined as the change in energy (in kj/mole) of a neutral atom (in the gaseous phase) when an electron is added. In chemistry and atomic physics, the electron affinity of an atom or molecule is. The electron affinity (ea) of an element. Electron Affinity Bromine Reaction.
From chem.libretexts.org
1.1.2.4 Electron Affinity Chemistry LibreTexts Electron Affinity Bromine Reaction The electron affinity (ea) of an element is the energy change that occurs when an electron is added to a gaseous atom to give an anion. Reactions 1 to 50, reactions 51 to 55; In chemistry and atomic physics, the electron affinity of an atom or molecule is. Electron affinity of bromine is 324.6 kj/mol. Electron affinity is defined as. Electron Affinity Bromine Reaction.
From www.nuclear-power.com
Bromine Electron Affinity Electronegativity Ionization Energy of Electron Affinity Bromine Reaction In general, elements with the most negative electron. The electron affinity (ea) of an element is the energy change that occurs when an electron is added to a gaseous atom to give an anion. Reactions 1 to 50, reactions 51 to 55; In chemistry and atomic physics, the electron affinity of an atom or molecule is. Electron affinity of bromine. Electron Affinity Bromine Reaction.
From general.chemistrysteps.com
Electron Affinity Chemistry Steps Electron Affinity Bromine Reaction In chemistry and atomic physics, the electron affinity of an atom or molecule is. In general, elements with the most negative electron. Reactions 1 to 50, reactions 51 to 55; Electron affinity is defined as the change in energy (in kj/mole) of a neutral atom (in the gaseous phase) when an electron is added. Electron affinity (d ecreases down the. Electron Affinity Bromine Reaction.
From www.animalia-life.club
Electron Configuration For Bromine Electron Affinity Bromine Reaction Electron affinity (d ecreases down the group) since the atomic size increases down the group, electron affinity generally decreases. Reactions 1 to 50, reactions 51 to 55; In chemistry and atomic physics, the electron affinity of an atom or molecule is. The electron affinity (ea) of an element is the energy change that occurs when an electron is added to. Electron Affinity Bromine Reaction.
From material-properties.org
Bromine Periodic Table and Atomic Properties Electron Affinity Bromine Reaction The electron affinity (ea) of an element is the energy change that occurs when an electron is added to a gaseous atom to give an anion. In general, elements with the most negative electron. In chemistry and atomic physics, the electron affinity of an atom or molecule is. Electron affinity is defined as the change in energy (in kj/mole) of. Electron Affinity Bromine Reaction.
From www.numerade.com
SOLVED Click the button that shows the correct relationship of the Electron Affinity Bromine Reaction Electron affinity (d ecreases down the group) since the atomic size increases down the group, electron affinity generally decreases. Reactions 1 to 50, reactions 51 to 55; The electron affinity (ea) of an element is the energy change that occurs when an electron is added to a gaseous atom to give an anion. In chemistry and atomic physics, the electron. Electron Affinity Bromine Reaction.
From www.chegg.com
Solved i. From the balanced reaction below which specie has Electron Affinity Bromine Reaction Electron affinity of bromine is 324.6 kj/mol. Electron affinity is defined as the change in energy (in kj/mole) of a neutral atom (in the gaseous phase) when an electron is added. The electron affinity (ea) of an element is the energy change that occurs when an electron is added to a gaseous atom to give an anion. Electron affinity (d. Electron Affinity Bromine Reaction.
From www.nagwa.com
Question Video Determining the Equation that Represents the Electron Electron Affinity Bromine Reaction Electron affinity (d ecreases down the group) since the atomic size increases down the group, electron affinity generally decreases. The electron affinity (ea) of an element is the energy change that occurs when an electron is added to a gaseous atom to give an anion. In general, elements with the most negative electron. Electron affinity of bromine is 324.6 kj/mol.. Electron Affinity Bromine Reaction.
From pressbooks.online.ucf.edu
4.4 Ionization energy and Electron Affinity Chemistry Fundamentals Electron Affinity Bromine Reaction Electron affinity of bromine is 324.6 kj/mol. The electron affinity (ea) of an element is the energy change that occurs when an electron is added to a gaseous atom to give an anion. Electron affinity is defined as the change in energy (in kj/mole) of a neutral atom (in the gaseous phase) when an electron is added. In chemistry and. Electron Affinity Bromine Reaction.
From ecurrencythailand.com
Which Of The Following Has Highest Electron Affinity Of Fluorine Electron Affinity Bromine Reaction In chemistry and atomic physics, the electron affinity of an atom or molecule is. Electron affinity of bromine is 324.6 kj/mol. Reactions 1 to 50, reactions 51 to 55; In general, elements with the most negative electron. The electron affinity (ea) of an element is the energy change that occurs when an electron is added to a gaseous atom to. Electron Affinity Bromine Reaction.
From exorwrmka.blob.core.windows.net
Bromine Of Electron Affinity at Curtis Phillips blog Electron Affinity Bromine Reaction The electron affinity (ea) of an element is the energy change that occurs when an electron is added to a gaseous atom to give an anion. In chemistry and atomic physics, the electron affinity of an atom or molecule is. Reactions 1 to 50, reactions 51 to 55; In general, elements with the most negative electron. Electron affinity is defined. Electron Affinity Bromine Reaction.
From www.toppr.com
The electron affinity of bromine atom is equal to the Electron Affinity Bromine Reaction The electron affinity (ea) of an element is the energy change that occurs when an electron is added to a gaseous atom to give an anion. Electron affinity is defined as the change in energy (in kj/mole) of a neutral atom (in the gaseous phase) when an electron is added. Electron affinity of bromine is 324.6 kj/mol. Electron affinity (d. Electron Affinity Bromine Reaction.
From ar.inspiredpencil.com
Electron Affinity Equation Electron Affinity Bromine Reaction Reactions 1 to 50, reactions 51 to 55; Electron affinity is defined as the change in energy (in kj/mole) of a neutral atom (in the gaseous phase) when an electron is added. In general, elements with the most negative electron. Electron affinity (d ecreases down the group) since the atomic size increases down the group, electron affinity generally decreases. In. Electron Affinity Bromine Reaction.
From mydigitalkemistry.com
Electron Affinity Trends Chemistry Digital Kemistry Best Online Electron Affinity Bromine Reaction Electron affinity of bromine is 324.6 kj/mol. Electron affinity is defined as the change in energy (in kj/mole) of a neutral atom (in the gaseous phase) when an electron is added. Reactions 1 to 50, reactions 51 to 55; In chemistry and atomic physics, the electron affinity of an atom or molecule is. Electron affinity (d ecreases down the group). Electron Affinity Bromine Reaction.
From studylib.net
Electron Attachment Enthalpy, ∆H Electron Affinity Bromine Reaction Reactions 1 to 50, reactions 51 to 55; In chemistry and atomic physics, the electron affinity of an atom or molecule is. Electron affinity of bromine is 324.6 kj/mol. Electron affinity is defined as the change in energy (in kj/mole) of a neutral atom (in the gaseous phase) when an electron is added. Electron affinity (d ecreases down the group). Electron Affinity Bromine Reaction.
From general.chemistrysteps.com
Electron Affinity Chemistry Steps Electron Affinity Bromine Reaction The electron affinity (ea) of an element is the energy change that occurs when an electron is added to a gaseous atom to give an anion. In chemistry and atomic physics, the electron affinity of an atom or molecule is. Electron affinity is defined as the change in energy (in kj/mole) of a neutral atom (in the gaseous phase) when. Electron Affinity Bromine Reaction.