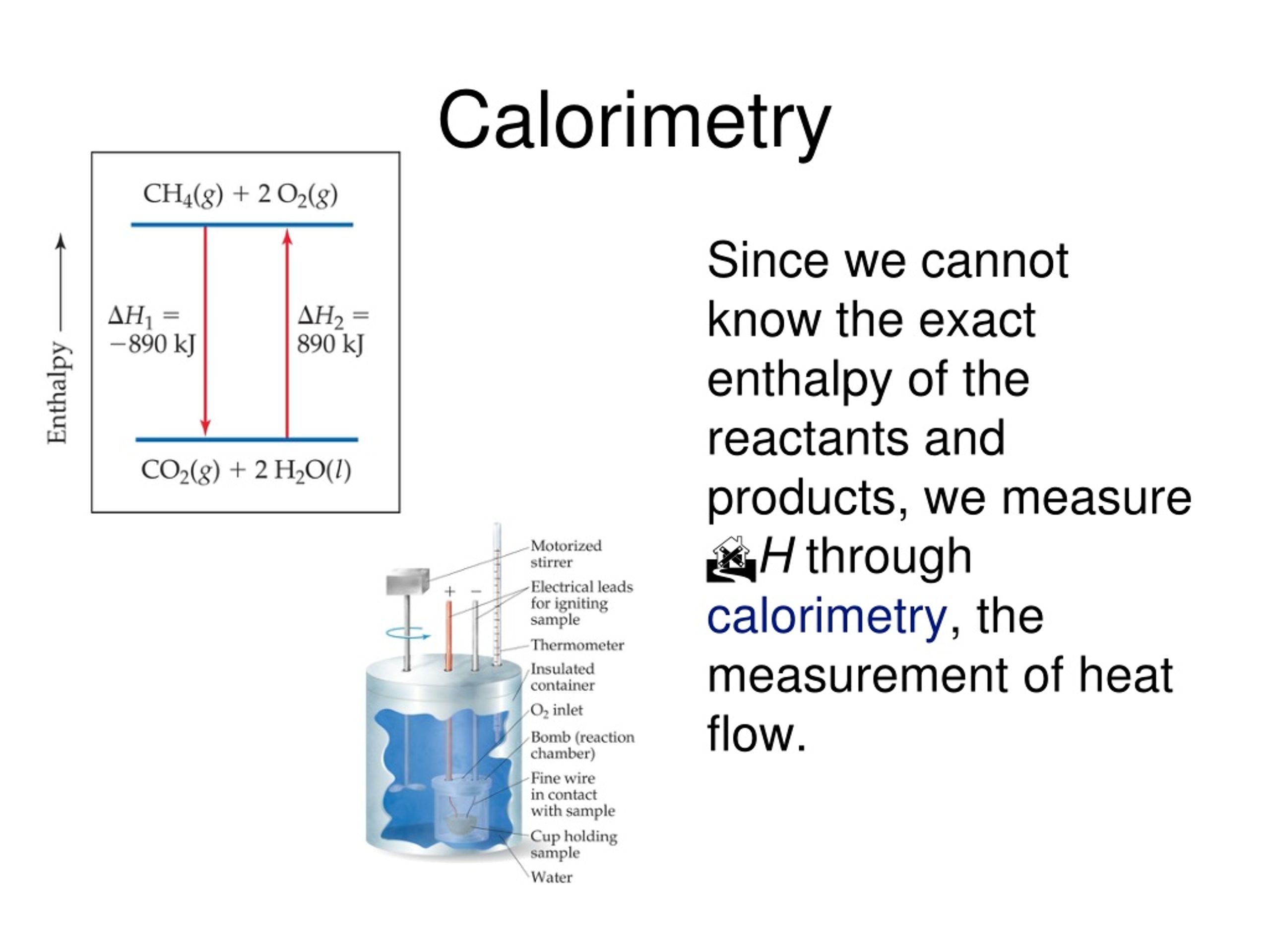
Calorimetry Real Life Examples . With a styrofoam calorimeter, the temperature of the substance inside the cup is measured, a reaction is allowed to take place, and afterward, the temperature is measured a second time. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to investigate its physical and chemical changes. Measuring the calories of everything you eat. It burns food in a sealed container, and uses the heat. Calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two substances in a calorimeter. Calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. Calorimetry is a method of measuring the heat transfer within a chemical reaction or other physical processes, such as a change between different states of matter. The term calorimetry comes from the latin calor (heat) and greek metron (measure), so it means measuring heat. A bomb calorimeter can calculate the amount of calories in foods we eat. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Apply the first law of thermodynamics to calorimetry. For example, when an exothermic reaction occurs in solution in a calorimeter, the heat produced by the reaction is absorbed by the solution, which increases its temperature. By knowing the change in. To determine the enthalpy, stability, heat capacity, and other thermochemical quantities, calorimetry is widely used.
from www.slideserve.com
Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. Calorimetry is a method of measuring the heat transfer within a chemical reaction or other physical processes, such as a change between different states of matter. Calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. Apply the first law of thermodynamics to calorimetry. Measuring the calories of everything you eat. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. The term calorimetry comes from the latin calor (heat) and greek metron (measure), so it means measuring heat. It burns food in a sealed container, and uses the heat. To determine the enthalpy, stability, heat capacity, and other thermochemical quantities, calorimetry is widely used. Calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two substances in a calorimeter.
PPT Calorimetry PowerPoint Presentation, free download ID373573
Calorimetry Real Life Examples A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. For example, when an exothermic reaction occurs in solution in a calorimeter, the heat produced by the reaction is absorbed by the solution, which increases its temperature. By knowing the change in. With a styrofoam calorimeter, the temperature of the substance inside the cup is measured, a reaction is allowed to take place, and afterward, the temperature is measured a second time. The term calorimetry comes from the latin calor (heat) and greek metron (measure), so it means measuring heat. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to investigate its physical and chemical changes. To determine the enthalpy, stability, heat capacity, and other thermochemical quantities, calorimetry is widely used. It burns food in a sealed container, and uses the heat. Calorimetry is a method of measuring the heat transfer within a chemical reaction or other physical processes, such as a change between different states of matter. Calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. Apply the first law of thermodynamics to calorimetry. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. A bomb calorimeter can calculate the amount of calories in foods we eat. Measuring the calories of everything you eat. Calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two substances in a calorimeter.
From www.slideserve.com
PPT Calorimetry C ontents Basic Concept Example Whiteboards Bomb Calorimetry Real Life Examples Measuring the calories of everything you eat. Calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two substances in a calorimeter. By knowing the change in. Calorimetry is a method of measuring the heat transfer within a chemical reaction or other physical processes, such as a change between different states of matter. For example, when an. Calorimetry Real Life Examples.
From klaxzllai.blob.core.windows.net
Calorimetry Equation Examples at Grace Smallwood blog Calorimetry Real Life Examples For example, when an exothermic reaction occurs in solution in a calorimeter, the heat produced by the reaction is absorbed by the solution, which increases its temperature. To determine the enthalpy, stability, heat capacity, and other thermochemical quantities, calorimetry is widely used. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. Calorimetry is. Calorimetry Real Life Examples.
From cepfukqw.blob.core.windows.net
When Is Calorimetry Used In Real Life at John Nelson blog Calorimetry Real Life Examples The term calorimetry comes from the latin calor (heat) and greek metron (measure), so it means measuring heat. Calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two substances in a calorimeter. Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to investigate its physical and chemical changes.. Calorimetry Real Life Examples.
From study.com
Bomb Calorimeter Uses, Equations & Examples Lesson Calorimetry Real Life Examples It burns food in a sealed container, and uses the heat. Calorimetry is a method of measuring the heat transfer within a chemical reaction or other physical processes, such as a change between different states of matter. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. Measuring the calories of everything you eat.. Calorimetry Real Life Examples.
From www.youtube.com
Calorimetry Example Calculation YouTube Calorimetry Real Life Examples A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two substances in a calorimeter. Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to investigate its physical and chemical. Calorimetry Real Life Examples.
From dxojkdaez.blob.core.windows.net
Calorimetry Physics Examples at Jeremy Wetmore blog Calorimetry Real Life Examples Calorimetry is a method of measuring the heat transfer within a chemical reaction or other physical processes, such as a change between different states of matter. Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to investigate its physical and chemical changes. Measuring the calories of everything you eat. By knowing the. Calorimetry Real Life Examples.
From users.highland.edu
Calorimetry Calorimetry Real Life Examples A bomb calorimeter can calculate the amount of calories in foods we eat. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. With a styrofoam calorimeter, the temperature of the substance inside the cup is measured, a reaction is allowed to take place, and afterward, the temperature is measured a second time. It. Calorimetry Real Life Examples.
From www.scribd.com
calorimetry Chemical Engineering Chemical Process Engineering Calorimetry Real Life Examples Calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. To determine the enthalpy, stability, heat capacity, and other thermochemical quantities, calorimetry is widely used. For example, when an exothermic reaction occurs in solution in a calorimeter, the heat produced by the reaction is absorbed by the solution, which increases its temperature. Calculate. Calorimetry Real Life Examples.
From maestrovirtuale.com
Calorimetria o que você estuda e aplicações Calorimetry Real Life Examples For example, when an exothermic reaction occurs in solution in a calorimeter, the heat produced by the reaction is absorbed by the solution, which increases its temperature. Apply the first law of thermodynamics to calorimetry. Calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two substances in a calorimeter. Calorimetry is a method of measuring the. Calorimetry Real Life Examples.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation, free download ID373573 Calorimetry Real Life Examples Measuring the calories of everything you eat. The term calorimetry comes from the latin calor (heat) and greek metron (measure), so it means measuring heat. With a styrofoam calorimeter, the temperature of the substance inside the cup is measured, a reaction is allowed to take place, and afterward, the temperature is measured a second time. A calorimeter is a device. Calorimetry Real Life Examples.
From cepfukqw.blob.core.windows.net
When Is Calorimetry Used In Real Life at John Nelson blog Calorimetry Real Life Examples Measuring the calories of everything you eat. The term calorimetry comes from the latin calor (heat) and greek metron (measure), so it means measuring heat. Calorimetry is a method of measuring the heat transfer within a chemical reaction or other physical processes, such as a change between different states of matter. Apply the first law of thermodynamics to calorimetry. Compare. Calorimetry Real Life Examples.
From www.slideserve.com
PPT CALORIMETRY PowerPoint Presentation, free download ID5767247 Calorimetry Real Life Examples It burns food in a sealed container, and uses the heat. Measuring the calories of everything you eat. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. Apply the first law of thermodynamics to calorimetry. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical. Calorimetry Real Life Examples.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation, free download ID5190363 Calorimetry Real Life Examples Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to investigate its physical and chemical changes. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. For example, when an exothermic reaction occurs in solution in a calorimeter, the heat produced by the. Calorimetry Real Life Examples.
From cepfukqw.blob.core.windows.net
When Is Calorimetry Used In Real Life at John Nelson blog Calorimetry Real Life Examples Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to investigate its physical and chemical changes. For example, when an exothermic reaction occurs in solution in a calorimeter, the heat produced by the reaction is absorbed by the solution, which increases its temperature. A calorimeter is a device used to measure the. Calorimetry Real Life Examples.
From www.slideserve.com
PPT Q = MCΔT Specific Heat & CALORIMETRY PowerPoint Presentation Calorimetry Real Life Examples Calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. Calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two substances in a calorimeter. It burns food in a sealed container, and uses the heat. Calorimetry is a method of measuring the heat transfer within a chemical reaction or. Calorimetry Real Life Examples.
From www.youtube.com
How To Solve Basic Calorimetry Problems in Chemistry YouTube Calorimetry Real Life Examples Measuring the calories of everything you eat. A bomb calorimeter can calculate the amount of calories in foods we eat. The term calorimetry comes from the latin calor (heat) and greek metron (measure), so it means measuring heat. Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to investigate its physical and. Calorimetry Real Life Examples.
From courses.lumenlearning.com
Calorimetry Chemistry for Majors Calorimetry Real Life Examples Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to investigate its physical and chemical changes. Apply the first law of thermodynamics to calorimetry. To determine the enthalpy, stability, heat capacity, and other thermochemical quantities, calorimetry is widely used. A bomb calorimeter can calculate the amount of calories in foods we eat.. Calorimetry Real Life Examples.
From psu.pb.unizin.org
Calorimetry (9.2) Chemistry 110 Calorimetry Real Life Examples Calorimetry is a method of measuring the heat transfer within a chemical reaction or other physical processes, such as a change between different states of matter. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. For example, when an exothermic reaction occurs in solution in a calorimeter, the heat produced by the reaction. Calorimetry Real Life Examples.
From www.slideserve.com
PPT Thermochemistry PowerPoint Presentation, free download ID2692866 Calorimetry Real Life Examples A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Measuring the calories of everything you eat. Calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. By knowing the change in. A bomb calorimeter can calculate the amount of calories in foods we. Calorimetry Real Life Examples.
From cepfukqw.blob.core.windows.net
When Is Calorimetry Used In Real Life at John Nelson blog Calorimetry Real Life Examples Apply the first law of thermodynamics to calorimetry. A bomb calorimeter can calculate the amount of calories in foods we eat. Calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. It burns food in a sealed. Calorimetry Real Life Examples.
From www.youtube.com
Physics 9.09g Calorimetry Example 2 YouTube Calorimetry Real Life Examples To determine the enthalpy, stability, heat capacity, and other thermochemical quantities, calorimetry is widely used. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. The term calorimetry comes from the latin calor (heat) and greek metron (measure), so it means measuring heat. A bomb calorimeter can calculate the amount of calories in foods. Calorimetry Real Life Examples.
From www.tec-science.com
Calorimeter to determine the specific heat capacities of liquids tec Calorimetry Real Life Examples Apply the first law of thermodynamics to calorimetry. Calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. The term calorimetry comes from the latin calor (heat) and greek metron (measure), so it means measuring heat. By knowing the change in. With a styrofoam calorimeter, the temperature of the substance inside the cup. Calorimetry Real Life Examples.
From www.slideshare.net
Lesson Enthalpy and Calorimetry Calorimetry Real Life Examples Calorimetry is a method of measuring the heat transfer within a chemical reaction or other physical processes, such as a change between different states of matter. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. Calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two substances in a calorimeter.. Calorimetry Real Life Examples.
From courses.lumenlearning.com
Calorimetry General Chemistry Calorimetry Real Life Examples To determine the enthalpy, stability, heat capacity, and other thermochemical quantities, calorimetry is widely used. Measuring the calories of everything you eat. For example, when an exothermic reaction occurs in solution in a calorimeter, the heat produced by the reaction is absorbed by the solution, which increases its temperature. It burns food in a sealed container, and uses the heat.. Calorimetry Real Life Examples.
From www.studypool.com
SOLUTION Chemistry cit u calorimetry definition examples sample Calorimetry Real Life Examples Calorimetry is a method of measuring the heat transfer within a chemical reaction or other physical processes, such as a change between different states of matter. Calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two substances in a calorimeter. For example, when an exothermic reaction occurs in solution in a calorimeter, the heat produced by. Calorimetry Real Life Examples.
From www.collegesearch.in
Principle of Calorimetry Definition, Formula, Principle, Types Calorimetry Real Life Examples Measuring the calories of everything you eat. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Apply the first law of thermodynamics to calorimetry. By knowing the change in. The term calorimetry comes from the latin calor (heat) and greek metron (measure), so it means measuring heat. For example, when. Calorimetry Real Life Examples.
From dxojkdaez.blob.core.windows.net
Calorimetry Physics Examples at Jeremy Wetmore blog Calorimetry Real Life Examples Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. To determine the enthalpy, stability, heat capacity, and other thermochemical quantities, calorimetry is widely used. The term calorimetry comes from the latin calor (heat) and greek metron (measure), so it means measuring heat. Calorimetry is a method of measuring the heat transfer within a. Calorimetry Real Life Examples.
From www.youtube.com
Physics 9.09b Calorimetry Example 1 YouTube Calorimetry Real Life Examples Calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. A bomb calorimeter can calculate the amount of calories in foods we eat. For example, when an exothermic reaction occurs in solution in a calorimeter, the heat produced by the reaction is absorbed by the solution, which increases its temperature. With a styrofoam. Calorimetry Real Life Examples.
From mmerevise.co.uk
Enthalpy Changes and Calorimetry MME Calorimetry Real Life Examples Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. Calorimetry is a method of measuring the heat transfer within a chemical reaction or other physical processes, such as a change between different states of matter. Calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two substances in a calorimeter.. Calorimetry Real Life Examples.
From www.youtube.com
CALORIMETRY EXAMPLE 3 YouTube Calorimetry Real Life Examples Measuring the calories of everything you eat. Calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two substances in a calorimeter. It burns food in a sealed container, and uses the heat. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. A calorimeter is a device used to measure. Calorimetry Real Life Examples.
From www.chemistrystudent.com
Calorimetry (ALevel) ChemistryStudent Calorimetry Real Life Examples For example, when an exothermic reaction occurs in solution in a calorimeter, the heat produced by the reaction is absorbed by the solution, which increases its temperature. It burns food in a sealed container, and uses the heat. The term calorimetry comes from the latin calor (heat) and greek metron (measure), so it means measuring heat. To determine the enthalpy,. Calorimetry Real Life Examples.
From cepfukqw.blob.core.windows.net
When Is Calorimetry Used In Real Life at John Nelson blog Calorimetry Real Life Examples The term calorimetry comes from the latin calor (heat) and greek metron (measure), so it means measuring heat. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. Calorimetry is a method of measuring the heat transfer within a chemical reaction or other physical processes, such as a change between different states of matter.. Calorimetry Real Life Examples.
From courses.lumenlearning.com
Calorimetry Chemistry Calorimetry Real Life Examples For example, when an exothermic reaction occurs in solution in a calorimeter, the heat produced by the reaction is absorbed by the solution, which increases its temperature. Calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two substances in a calorimeter. The term calorimetry comes from the latin calor (heat) and greek metron (measure), so it. Calorimetry Real Life Examples.
From www.youtube.com
Calorimetry examples YouTube Calorimetry Real Life Examples A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. To determine the enthalpy, stability, heat capacity, and other thermochemical quantities, calorimetry is widely used. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. For example, when an exothermic reaction occurs in solution in. Calorimetry Real Life Examples.
From www.slideserve.com
PPT Calorimeters & Calorimetry PowerPoint Presentation, free download Calorimetry Real Life Examples The term calorimetry comes from the latin calor (heat) and greek metron (measure), so it means measuring heat. For example, when an exothermic reaction occurs in solution in a calorimeter, the heat produced by the reaction is absorbed by the solution, which increases its temperature. Calorimetry is a branch of science concerned with measuring a body’s state in terms of. Calorimetry Real Life Examples.