Iron Iii Oxide And Sodium Hydroxide . Sodium hydroxide is a soluble base (alkali) and iron (iii) oxide is a base too. Iron salts react with dilute alkali solutions, specifically the hydroxide ions, to form either iron(ii) hydroxide or iron(iii) hydroxide depending on the starting iron salt. The precipitate again changes colour as the iron(ii) hydroxide complex is oxidised by the air to iron(iii) hydroxide. Iron is very easily oxidized under alkaline conditions. The reaction looks just the same as when you add sodium. Oxygen in the air oxidizes the iron(ii) hydroxide precipitate to iron(iii) hydroxide. The short answer is no. Both precipitates are insoluble in excess aqueous ammonia. An acid will react with metal oxides. Iron (ii) hydroxide quickly oxidizes to fe(oh) 3 in the presence of air or other oxidizing agents. Iron(iii) oxide reacts with strong bases, like. Iron (iii) oxide tablets were electrolytically reduced to iron in molten sodium hydroxide at 530 °c and recovered to produce.
from stock.adobe.com
Iron salts react with dilute alkali solutions, specifically the hydroxide ions, to form either iron(ii) hydroxide or iron(iii) hydroxide depending on the starting iron salt. Sodium hydroxide is a soluble base (alkali) and iron (iii) oxide is a base too. The reaction looks just the same as when you add sodium. Oxygen in the air oxidizes the iron(ii) hydroxide precipitate to iron(iii) hydroxide. The short answer is no. Iron (iii) oxide tablets were electrolytically reduced to iron in molten sodium hydroxide at 530 °c and recovered to produce. Iron (ii) hydroxide quickly oxidizes to fe(oh) 3 in the presence of air or other oxidizing agents. The precipitate again changes colour as the iron(ii) hydroxide complex is oxidised by the air to iron(iii) hydroxide. Iron is very easily oxidized under alkaline conditions. Iron(iii) oxide reacts with strong bases, like.
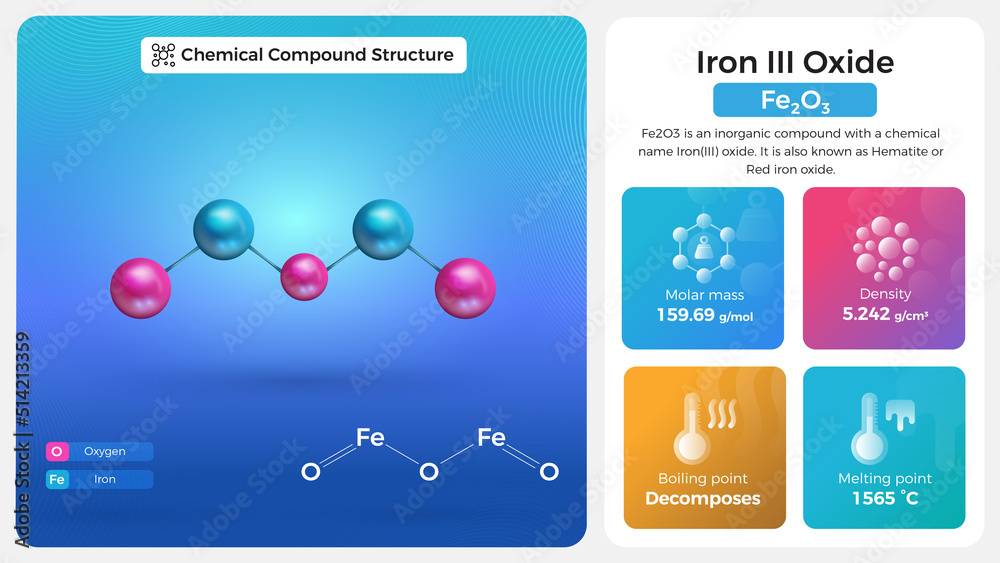
iron III Oxide Properties and Chemical Compound Structure Stock Vector
Iron Iii Oxide And Sodium Hydroxide The short answer is no. The short answer is no. Oxygen in the air oxidizes the iron(ii) hydroxide precipitate to iron(iii) hydroxide. An acid will react with metal oxides. Iron (iii) oxide tablets were electrolytically reduced to iron in molten sodium hydroxide at 530 °c and recovered to produce. Iron (ii) hydroxide quickly oxidizes to fe(oh) 3 in the presence of air or other oxidizing agents. Sodium hydroxide is a soluble base (alkali) and iron (iii) oxide is a base too. Iron salts react with dilute alkali solutions, specifically the hydroxide ions, to form either iron(ii) hydroxide or iron(iii) hydroxide depending on the starting iron salt. Iron(iii) oxide reacts with strong bases, like. Iron is very easily oxidized under alkaline conditions. The precipitate again changes colour as the iron(ii) hydroxide complex is oxidised by the air to iron(iii) hydroxide. The reaction looks just the same as when you add sodium. Both precipitates are insoluble in excess aqueous ammonia.
From stock.adobe.com
iron III Oxide Properties and Chemical Compound Structure Stock Vector Iron Iii Oxide And Sodium Hydroxide The precipitate again changes colour as the iron(ii) hydroxide complex is oxidised by the air to iron(iii) hydroxide. Oxygen in the air oxidizes the iron(ii) hydroxide precipitate to iron(iii) hydroxide. Iron(iii) oxide reacts with strong bases, like. Iron (iii) oxide tablets were electrolytically reduced to iron in molten sodium hydroxide at 530 °c and recovered to produce. Iron is very. Iron Iii Oxide And Sodium Hydroxide.
From slideplayer.com
Ionic Compounds Naming ppt download Iron Iii Oxide And Sodium Hydroxide Iron salts react with dilute alkali solutions, specifically the hydroxide ions, to form either iron(ii) hydroxide or iron(iii) hydroxide depending on the starting iron salt. Iron(iii) oxide reacts with strong bases, like. The short answer is no. Oxygen in the air oxidizes the iron(ii) hydroxide precipitate to iron(iii) hydroxide. Iron (ii) hydroxide quickly oxidizes to fe(oh) 3 in the presence. Iron Iii Oxide And Sodium Hydroxide.
From www.sciencephoto.com
Iron (III) hydroxide precipitate Stock Image A500/0413 Science Iron Iii Oxide And Sodium Hydroxide Iron (ii) hydroxide quickly oxidizes to fe(oh) 3 in the presence of air or other oxidizing agents. Iron (iii) oxide tablets were electrolytically reduced to iron in molten sodium hydroxide at 530 °c and recovered to produce. The short answer is no. Sodium hydroxide is a soluble base (alkali) and iron (iii) oxide is a base too. An acid will. Iron Iii Oxide And Sodium Hydroxide.
From www.youtube.com
The Reaction Between Iron (III) Nitrate and Sodium Hydroxide YouTube Iron Iii Oxide And Sodium Hydroxide The reaction looks just the same as when you add sodium. An acid will react with metal oxides. Iron salts react with dilute alkali solutions, specifically the hydroxide ions, to form either iron(ii) hydroxide or iron(iii) hydroxide depending on the starting iron salt. Sodium hydroxide is a soluble base (alkali) and iron (iii) oxide is a base too. Iron is. Iron Iii Oxide And Sodium Hydroxide.
From tecnico.aspillagahornauer.cl
Iron(III) Oxidehydroxide Wikipedia, 60 OFF Iron Iii Oxide And Sodium Hydroxide Oxygen in the air oxidizes the iron(ii) hydroxide precipitate to iron(iii) hydroxide. Iron (iii) oxide tablets were electrolytically reduced to iron in molten sodium hydroxide at 530 °c and recovered to produce. Both precipitates are insoluble in excess aqueous ammonia. Sodium hydroxide is a soluble base (alkali) and iron (iii) oxide is a base too. The precipitate again changes colour. Iron Iii Oxide And Sodium Hydroxide.
From fyojjdtpa.blob.core.windows.net
Iron Iii Oxide And Sodium Hydroxide at Laura Yeager blog Iron Iii Oxide And Sodium Hydroxide Iron is very easily oxidized under alkaline conditions. Iron (ii) hydroxide quickly oxidizes to fe(oh) 3 in the presence of air or other oxidizing agents. Iron (iii) oxide tablets were electrolytically reduced to iron in molten sodium hydroxide at 530 °c and recovered to produce. Iron(iii) oxide reacts with strong bases, like. The precipitate again changes colour as the iron(ii). Iron Iii Oxide And Sodium Hydroxide.
From www.youtube.com
How to Write the Formula for Iron (III) hydroxide YouTube Iron Iii Oxide And Sodium Hydroxide Both precipitates are insoluble in excess aqueous ammonia. Iron salts react with dilute alkali solutions, specifically the hydroxide ions, to form either iron(ii) hydroxide or iron(iii) hydroxide depending on the starting iron salt. Iron (iii) oxide tablets were electrolytically reduced to iron in molten sodium hydroxide at 530 °c and recovered to produce. The reaction looks just the same as. Iron Iii Oxide And Sodium Hydroxide.
From fyojjdtpa.blob.core.windows.net
Iron Iii Oxide And Sodium Hydroxide at Laura Yeager blog Iron Iii Oxide And Sodium Hydroxide Iron (ii) hydroxide quickly oxidizes to fe(oh) 3 in the presence of air or other oxidizing agents. The precipitate again changes colour as the iron(ii) hydroxide complex is oxidised by the air to iron(iii) hydroxide. The short answer is no. The reaction looks just the same as when you add sodium. Iron is very easily oxidized under alkaline conditions. Iron. Iron Iii Oxide And Sodium Hydroxide.
From www.slideserve.com
PPT Nomenclature Review PowerPoint Presentation, free download ID Iron Iii Oxide And Sodium Hydroxide Iron salts react with dilute alkali solutions, specifically the hydroxide ions, to form either iron(ii) hydroxide or iron(iii) hydroxide depending on the starting iron salt. The precipitate again changes colour as the iron(ii) hydroxide complex is oxidised by the air to iron(iii) hydroxide. Iron is very easily oxidized under alkaline conditions. Iron (iii) oxide tablets were electrolytically reduced to iron. Iron Iii Oxide And Sodium Hydroxide.
From www.youtube.com
Gas stoichiometry of iron(III) hydroxide YouTube Iron Iii Oxide And Sodium Hydroxide The short answer is no. Sodium hydroxide is a soluble base (alkali) and iron (iii) oxide is a base too. Iron salts react with dilute alkali solutions, specifically the hydroxide ions, to form either iron(ii) hydroxide or iron(iii) hydroxide depending on the starting iron salt. The reaction looks just the same as when you add sodium. Both precipitates are insoluble. Iron Iii Oxide And Sodium Hydroxide.
From davis-well-rodgers.blogspot.com
Iron Iii Chloride and Sodium Hydroxide Ionic Equation DaviswellRodgers Iron Iii Oxide And Sodium Hydroxide Oxygen in the air oxidizes the iron(ii) hydroxide precipitate to iron(iii) hydroxide. The reaction looks just the same as when you add sodium. Iron (ii) hydroxide quickly oxidizes to fe(oh) 3 in the presence of air or other oxidizing agents. An acid will react with metal oxides. Iron is very easily oxidized under alkaline conditions. Sodium hydroxide is a soluble. Iron Iii Oxide And Sodium Hydroxide.
From klayacqjg.blob.core.windows.net
Iron Iii Oxide at Florencio Carnell blog Iron Iii Oxide And Sodium Hydroxide The precipitate again changes colour as the iron(ii) hydroxide complex is oxidised by the air to iron(iii) hydroxide. Iron(iii) oxide reacts with strong bases, like. Sodium hydroxide is a soluble base (alkali) and iron (iii) oxide is a base too. Iron (iii) oxide tablets were electrolytically reduced to iron in molten sodium hydroxide at 530 °c and recovered to produce.. Iron Iii Oxide And Sodium Hydroxide.
From www.youtube.com
Iron III hydroxide precipitate, Chemistry Experiment YouTube Iron Iii Oxide And Sodium Hydroxide Iron is very easily oxidized under alkaline conditions. Both precipitates are insoluble in excess aqueous ammonia. Iron(iii) oxide reacts with strong bases, like. Iron salts react with dilute alkali solutions, specifically the hydroxide ions, to form either iron(ii) hydroxide or iron(iii) hydroxide depending on the starting iron salt. Iron (iii) oxide tablets were electrolytically reduced to iron in molten sodium. Iron Iii Oxide And Sodium Hydroxide.
From davis-well-rodgers.blogspot.com
Iron Iii Chloride and Sodium Hydroxide Ionic Equation DaviswellRodgers Iron Iii Oxide And Sodium Hydroxide The short answer is no. Iron (iii) oxide tablets were electrolytically reduced to iron in molten sodium hydroxide at 530 °c and recovered to produce. Oxygen in the air oxidizes the iron(ii) hydroxide precipitate to iron(iii) hydroxide. Iron is very easily oxidized under alkaline conditions. Iron (ii) hydroxide quickly oxidizes to fe(oh) 3 in the presence of air or other. Iron Iii Oxide And Sodium Hydroxide.
From testbook.com
Iron (III) Hydroxide Formula Its Structure, Preparation & Uses Iron Iii Oxide And Sodium Hydroxide Oxygen in the air oxidizes the iron(ii) hydroxide precipitate to iron(iii) hydroxide. The precipitate again changes colour as the iron(ii) hydroxide complex is oxidised by the air to iron(iii) hydroxide. An acid will react with metal oxides. Iron (iii) oxide tablets were electrolytically reduced to iron in molten sodium hydroxide at 530 °c and recovered to produce. Sodium hydroxide is. Iron Iii Oxide And Sodium Hydroxide.
From www.coursehero.com
[Solved] When aqueous an solutions of iron(III) sulfate and sodium Iron Iii Oxide And Sodium Hydroxide Iron (ii) hydroxide quickly oxidizes to fe(oh) 3 in the presence of air or other oxidizing agents. An acid will react with metal oxides. Oxygen in the air oxidizes the iron(ii) hydroxide precipitate to iron(iii) hydroxide. The reaction looks just the same as when you add sodium. Sodium hydroxide is a soluble base (alkali) and iron (iii) oxide is a. Iron Iii Oxide And Sodium Hydroxide.
From www.nagwa.com
Question Video Identifying Iron Oxide Produced from the Reaction of Iron Iii Oxide And Sodium Hydroxide The short answer is no. Iron(iii) oxide reacts with strong bases, like. Iron (ii) hydroxide quickly oxidizes to fe(oh) 3 in the presence of air or other oxidizing agents. An acid will react with metal oxides. The reaction looks just the same as when you add sodium. Iron salts react with dilute alkali solutions, specifically the hydroxide ions, to form. Iron Iii Oxide And Sodium Hydroxide.
From fyojjdtpa.blob.core.windows.net
Iron Iii Oxide And Sodium Hydroxide at Laura Yeager blog Iron Iii Oxide And Sodium Hydroxide Iron (ii) hydroxide quickly oxidizes to fe(oh) 3 in the presence of air or other oxidizing agents. Both precipitates are insoluble in excess aqueous ammonia. Iron (iii) oxide tablets were electrolytically reduced to iron in molten sodium hydroxide at 530 °c and recovered to produce. Oxygen in the air oxidizes the iron(ii) hydroxide precipitate to iron(iii) hydroxide. Sodium hydroxide is. Iron Iii Oxide And Sodium Hydroxide.
From www.shutterstock.com
Iron Iii Hydroxide Formula Handwritten Chemical Stock Illustration Iron Iii Oxide And Sodium Hydroxide The precipitate again changes colour as the iron(ii) hydroxide complex is oxidised by the air to iron(iii) hydroxide. The short answer is no. Sodium hydroxide is a soluble base (alkali) and iron (iii) oxide is a base too. Iron salts react with dilute alkali solutions, specifically the hydroxide ions, to form either iron(ii) hydroxide or iron(iii) hydroxide depending on the. Iron Iii Oxide And Sodium Hydroxide.
From www.sciencephoto.com
Iron(III) Oxide Stock Image C030/8153 Science Photo Library Iron Iii Oxide And Sodium Hydroxide The reaction looks just the same as when you add sodium. Iron (iii) oxide tablets were electrolytically reduced to iron in molten sodium hydroxide at 530 °c and recovered to produce. The precipitate again changes colour as the iron(ii) hydroxide complex is oxidised by the air to iron(iii) hydroxide. Iron is very easily oxidized under alkaline conditions. An acid will. Iron Iii Oxide And Sodium Hydroxide.
From fphoto.photoshelter.com
science chemistry precipitation reaction Fundamental Photographs Iron Iii Oxide And Sodium Hydroxide Iron salts react with dilute alkali solutions, specifically the hydroxide ions, to form either iron(ii) hydroxide or iron(iii) hydroxide depending on the starting iron salt. The short answer is no. Iron (iii) oxide tablets were electrolytically reduced to iron in molten sodium hydroxide at 530 °c and recovered to produce. Oxygen in the air oxidizes the iron(ii) hydroxide precipitate to. Iron Iii Oxide And Sodium Hydroxide.
From fphoto.photoshelter.com
science chemistry precipitation reaction iron iii hydroxide Iron Iii Oxide And Sodium Hydroxide Iron is very easily oxidized under alkaline conditions. The reaction looks just the same as when you add sodium. Sodium hydroxide is a soluble base (alkali) and iron (iii) oxide is a base too. An acid will react with metal oxides. Iron salts react with dilute alkali solutions, specifically the hydroxide ions, to form either iron(ii) hydroxide or iron(iii) hydroxide. Iron Iii Oxide And Sodium Hydroxide.
From www.numerade.com
SOLVED '11. Sodium perchlorate and iron can combine; as shown below Iron Iii Oxide And Sodium Hydroxide Iron (iii) oxide tablets were electrolytically reduced to iron in molten sodium hydroxide at 530 °c and recovered to produce. Iron(iii) oxide reacts with strong bases, like. Iron (ii) hydroxide quickly oxidizes to fe(oh) 3 in the presence of air or other oxidizing agents. Iron salts react with dilute alkali solutions, specifically the hydroxide ions, to form either iron(ii) hydroxide. Iron Iii Oxide And Sodium Hydroxide.
From www.researchgate.net
Mineral pigments in the iron(III) oxide/hydroxide system. Download Iron Iii Oxide And Sodium Hydroxide Iron (ii) hydroxide quickly oxidizes to fe(oh) 3 in the presence of air or other oxidizing agents. Oxygen in the air oxidizes the iron(ii) hydroxide precipitate to iron(iii) hydroxide. The short answer is no. The precipitate again changes colour as the iron(ii) hydroxide complex is oxidised by the air to iron(iii) hydroxide. The reaction looks just the same as when. Iron Iii Oxide And Sodium Hydroxide.
From pixels.com
Sodium Hydroxide And Iron IIi Chloride Photograph by Martyn F Iron Iii Oxide And Sodium Hydroxide Oxygen in the air oxidizes the iron(ii) hydroxide precipitate to iron(iii) hydroxide. Both precipitates are insoluble in excess aqueous ammonia. Iron (iii) oxide tablets were electrolytically reduced to iron in molten sodium hydroxide at 530 °c and recovered to produce. Iron salts react with dilute alkali solutions, specifically the hydroxide ions, to form either iron(ii) hydroxide or iron(iii) hydroxide depending. Iron Iii Oxide And Sodium Hydroxide.
From www.youtube.com
Reaction of Iron(III) Nitrate (Fe(NO3)3) and Sodium hydroxide (NaOH Iron Iii Oxide And Sodium Hydroxide Sodium hydroxide is a soluble base (alkali) and iron (iii) oxide is a base too. Iron (iii) oxide tablets were electrolytically reduced to iron in molten sodium hydroxide at 530 °c and recovered to produce. The short answer is no. Both precipitates are insoluble in excess aqueous ammonia. The reaction looks just the same as when you add sodium. An. Iron Iii Oxide And Sodium Hydroxide.
From www.numerade.com
SOLVED A student writes the following incorrect chemical equation for Iron Iii Oxide And Sodium Hydroxide Iron is very easily oxidized under alkaline conditions. Sodium hydroxide is a soluble base (alkali) and iron (iii) oxide is a base too. Iron (ii) hydroxide quickly oxidizes to fe(oh) 3 in the presence of air or other oxidizing agents. The reaction looks just the same as when you add sodium. The precipitate again changes colour as the iron(ii) hydroxide. Iron Iii Oxide And Sodium Hydroxide.
From www.youtube.com
Redox Reaction Oxidation of Iron (II) to Iron (III) YouTube Iron Iii Oxide And Sodium Hydroxide Iron (ii) hydroxide quickly oxidizes to fe(oh) 3 in the presence of air or other oxidizing agents. Iron is very easily oxidized under alkaline conditions. The reaction looks just the same as when you add sodium. Iron(iii) oxide reacts with strong bases, like. Iron (iii) oxide tablets were electrolytically reduced to iron in molten sodium hydroxide at 530 °c and. Iron Iii Oxide And Sodium Hydroxide.
From www.numerade.com
SOLVED The thermal of iron(III) hydroxide to produce Iron Iii Oxide And Sodium Hydroxide Sodium hydroxide is a soluble base (alkali) and iron (iii) oxide is a base too. Oxygen in the air oxidizes the iron(ii) hydroxide precipitate to iron(iii) hydroxide. The precipitate again changes colour as the iron(ii) hydroxide complex is oxidised by the air to iron(iii) hydroxide. Iron is very easily oxidized under alkaline conditions. The reaction looks just the same as. Iron Iii Oxide And Sodium Hydroxide.
From www.youtube.com
The reaction of iron(III) solution with sodium hydroxide and ammonia Iron Iii Oxide And Sodium Hydroxide The reaction looks just the same as when you add sodium. Iron (ii) hydroxide quickly oxidizes to fe(oh) 3 in the presence of air or other oxidizing agents. Oxygen in the air oxidizes the iron(ii) hydroxide precipitate to iron(iii) hydroxide. The short answer is no. Iron(iii) oxide reacts with strong bases, like. Iron is very easily oxidized under alkaline conditions.. Iron Iii Oxide And Sodium Hydroxide.
From www.youtube.com
Addition sodium hydroxide to iron (II) chloride and iron (III) chloride Iron Iii Oxide And Sodium Hydroxide Oxygen in the air oxidizes the iron(ii) hydroxide precipitate to iron(iii) hydroxide. Iron salts react with dilute alkali solutions, specifically the hydroxide ions, to form either iron(ii) hydroxide or iron(iii) hydroxide depending on the starting iron salt. The reaction looks just the same as when you add sodium. Both precipitates are insoluble in excess aqueous ammonia. Iron is very easily. Iron Iii Oxide And Sodium Hydroxide.
From www.fishersci.se
Iron(III) hydroxide, alphaphase, 99+, Thermo Scientific Chemicals Iron Iii Oxide And Sodium Hydroxide Both precipitates are insoluble in excess aqueous ammonia. Iron salts react with dilute alkali solutions, specifically the hydroxide ions, to form either iron(ii) hydroxide or iron(iii) hydroxide depending on the starting iron salt. Iron (ii) hydroxide quickly oxidizes to fe(oh) 3 in the presence of air or other oxidizing agents. The precipitate again changes colour as the iron(ii) hydroxide complex. Iron Iii Oxide And Sodium Hydroxide.
From www.numerade.com
SOLVED In water, iron(III) chloride reacts with sodium hydroxide Iron Iii Oxide And Sodium Hydroxide Iron (iii) oxide tablets were electrolytically reduced to iron in molten sodium hydroxide at 530 °c and recovered to produce. Both precipitates are insoluble in excess aqueous ammonia. Iron salts react with dilute alkali solutions, specifically the hydroxide ions, to form either iron(ii) hydroxide or iron(iii) hydroxide depending on the starting iron salt. Iron is very easily oxidized under alkaline. Iron Iii Oxide And Sodium Hydroxide.
From www.kolabshop.com
IRON(III) OXIDE, HYDRATED, CATALYST GRA& (371254) 코랩샵 KOLAB 연구용 기자재 Iron Iii Oxide And Sodium Hydroxide Oxygen in the air oxidizes the iron(ii) hydroxide precipitate to iron(iii) hydroxide. An acid will react with metal oxides. Both precipitates are insoluble in excess aqueous ammonia. Iron salts react with dilute alkali solutions, specifically the hydroxide ions, to form either iron(ii) hydroxide or iron(iii) hydroxide depending on the starting iron salt. Iron (iii) oxide tablets were electrolytically reduced to. Iron Iii Oxide And Sodium Hydroxide.
From learning-ezra.blogspot.com
Iron Iii Bromide And Sodium Hydroxide 22+ Pages Summary in Doc [1.6mb Iron Iii Oxide And Sodium Hydroxide Iron (iii) oxide tablets were electrolytically reduced to iron in molten sodium hydroxide at 530 °c and recovered to produce. Oxygen in the air oxidizes the iron(ii) hydroxide precipitate to iron(iii) hydroxide. Both precipitates are insoluble in excess aqueous ammonia. Iron is very easily oxidized under alkaline conditions. The reaction looks just the same as when you add sodium. The. Iron Iii Oxide And Sodium Hydroxide.