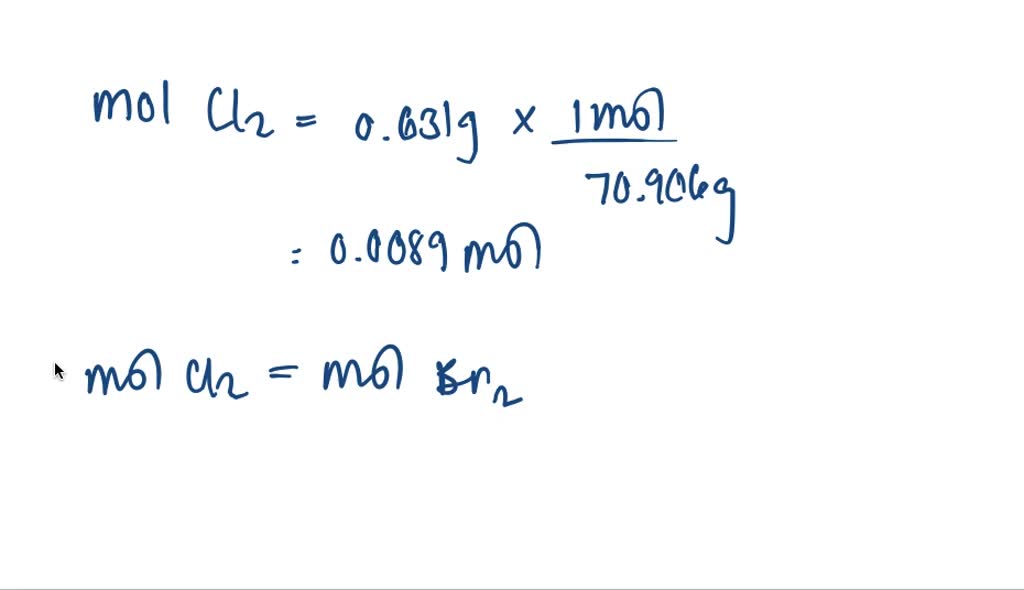Chlorine + Bromine Equation . Cl + br = clbr is a synthesis reaction where one mole of chlorine [cl] and one mole of bromine [br]. Chlorine will displace bromine or iodine from an aqueous solution of the metal halide: When chloromethane (or methylchloride) reacts with cl 2, another hydrogen is replaced by chlorine atom to give. When chlorine (as a gas or dissolved in water) is added to sodium bromide solution, the chlorine takes the place of the. Cl 2 (aq) + 2ki (aq) → 2kcl (aq) + i 2 (aq) the reaction mixture turns darker and iodine solution forms. Cl 2 (aq) + 2ki(aq) → i 2 (aq) +. Cl 2 (g) + 2kbr(aq) →. Chlorine + potassium bromide → bromine + potassium chloride symbol equation: In the displacement reactions chlorine displaces both bromine and iodine from their compounds and bromine displaces iodine. Cl 2 (aq) + 2kbr (aq) → 2kcl (aq) + br 2. Chlorine + bromine = bromine monochloride.
from www.numerade.com
Cl 2 (aq) + 2ki (aq) → 2kcl (aq) + i 2 (aq) the reaction mixture turns darker and iodine solution forms. Cl 2 (aq) + 2kbr (aq) → 2kcl (aq) + br 2. Cl 2 (g) + 2kbr(aq) →. In the displacement reactions chlorine displaces both bromine and iodine from their compounds and bromine displaces iodine. When chloromethane (or methylchloride) reacts with cl 2, another hydrogen is replaced by chlorine atom to give. Cl 2 (aq) + 2ki(aq) → i 2 (aq) +. Chlorine + potassium bromide → bromine + potassium chloride symbol equation: Chlorine + bromine = bromine monochloride. When chlorine (as a gas or dissolved in water) is added to sodium bromide solution, the chlorine takes the place of the. Chlorine will displace bromine or iodine from an aqueous solution of the metal halide:
SOLVEDGaseous chlorine will displace bromide ion from an aqueous
Chlorine + Bromine Equation Cl 2 (aq) + 2ki(aq) → i 2 (aq) +. Chlorine will displace bromine or iodine from an aqueous solution of the metal halide: Cl + br = clbr is a synthesis reaction where one mole of chlorine [cl] and one mole of bromine [br]. When chloromethane (or methylchloride) reacts with cl 2, another hydrogen is replaced by chlorine atom to give. In the displacement reactions chlorine displaces both bromine and iodine from their compounds and bromine displaces iodine. Chlorine + potassium bromide → bromine + potassium chloride symbol equation: Cl 2 (g) + 2kbr(aq) →. Cl 2 (aq) + 2ki (aq) → 2kcl (aq) + i 2 (aq) the reaction mixture turns darker and iodine solution forms. Chlorine + bromine = bromine monochloride. Cl 2 (aq) + 2ki(aq) → i 2 (aq) +. When chlorine (as a gas or dissolved in water) is added to sodium bromide solution, the chlorine takes the place of the. Cl 2 (aq) + 2kbr (aq) → 2kcl (aq) + br 2.
From www.youtube.com
NaBr+Cl2=NaCl+Br2 Balanced EquationSodium Bromide+Chlorine=Sodium Chlorine + Bromine Equation When chlorine (as a gas or dissolved in water) is added to sodium bromide solution, the chlorine takes the place of the. Cl 2 (aq) + 2ki(aq) → i 2 (aq) +. Chlorine + bromine = bromine monochloride. Cl 2 (aq) + 2kbr (aq) → 2kcl (aq) + br 2. Chlorine will displace bromine or iodine from an aqueous solution. Chlorine + Bromine Equation.
From www.numerade.com
SOLVED Chlorine gas reacts with aqueous sodium bromide to produce Chlorine + Bromine Equation When chloromethane (or methylchloride) reacts with cl 2, another hydrogen is replaced by chlorine atom to give. Cl 2 (aq) + 2ki(aq) → i 2 (aq) +. Chlorine + potassium bromide → bromine + potassium chloride symbol equation: Cl + br = clbr is a synthesis reaction where one mole of chlorine [cl] and one mole of bromine [br]. Cl. Chlorine + Bromine Equation.
From studylib.net
Displacement reactions Reactions of chlorine Chlorine + Bromine Equation Cl 2 (g) + 2kbr(aq) →. Chlorine will displace bromine or iodine from an aqueous solution of the metal halide: Chlorine + potassium bromide → bromine + potassium chloride symbol equation: When chlorine (as a gas or dissolved in water) is added to sodium bromide solution, the chlorine takes the place of the. Cl 2 (aq) + 2ki (aq) →. Chlorine + Bromine Equation.
From www.alamy.com
Molecular Model of Bromine (Br2) Molecule. Vector Illustration Stock Chlorine + Bromine Equation Cl 2 (aq) + 2kbr (aq) → 2kcl (aq) + br 2. Cl 2 (aq) + 2ki (aq) → 2kcl (aq) + i 2 (aq) the reaction mixture turns darker and iodine solution forms. Chlorine + potassium bromide → bromine + potassium chloride symbol equation: Cl 2 (g) + 2kbr(aq) →. When chlorine (as a gas or dissolved in water). Chlorine + Bromine Equation.
From www.diffzy.com
Bromine vs. Chlorine What's The Difference In Tabular Form, Points Chlorine + Bromine Equation Cl 2 (g) + 2kbr(aq) →. When chloromethane (or methylchloride) reacts with cl 2, another hydrogen is replaced by chlorine atom to give. Cl 2 (aq) + 2ki(aq) → i 2 (aq) +. When chlorine (as a gas or dissolved in water) is added to sodium bromide solution, the chlorine takes the place of the. Cl + br = clbr. Chlorine + Bromine Equation.
From www.slideserve.com
PPT Factoring Isotope Patterns Chlorine and Bromine with nitrogen Chlorine + Bromine Equation When chloromethane (or methylchloride) reacts with cl 2, another hydrogen is replaced by chlorine atom to give. In the displacement reactions chlorine displaces both bromine and iodine from their compounds and bromine displaces iodine. Chlorine + potassium bromide → bromine + potassium chloride symbol equation: Cl 2 (aq) + 2ki (aq) → 2kcl (aq) + i 2 (aq) the reaction. Chlorine + Bromine Equation.
From slideplayer.com
Biology Dr. Altstiel Naples American High School ppt download Chlorine + Bromine Equation Chlorine will displace bromine or iodine from an aqueous solution of the metal halide: When chlorine (as a gas or dissolved in water) is added to sodium bromide solution, the chlorine takes the place of the. Cl 2 (g) + 2kbr(aq) →. In the displacement reactions chlorine displaces both bromine and iodine from their compounds and bromine displaces iodine. Cl. Chlorine + Bromine Equation.
From www.chegg.com
Solved Chlorine and bromine react in the dark with alkenes. Chlorine + Bromine Equation Cl 2 (aq) + 2ki (aq) → 2kcl (aq) + i 2 (aq) the reaction mixture turns darker and iodine solution forms. When chlorine (as a gas or dissolved in water) is added to sodium bromide solution, the chlorine takes the place of the. Chlorine + bromine = bromine monochloride. Cl 2 (aq) + 2kbr (aq) → 2kcl (aq) +. Chlorine + Bromine Equation.
From www.numerade.com
SOLVED Potassium bromide + chlorine = potassium chloride + bromine Chlorine + Bromine Equation Chlorine will displace bromine or iodine from an aqueous solution of the metal halide: Cl 2 (aq) + 2ki(aq) → i 2 (aq) +. When chlorine (as a gas or dissolved in water) is added to sodium bromide solution, the chlorine takes the place of the. When chloromethane (or methylchloride) reacts with cl 2, another hydrogen is replaced by chlorine. Chlorine + Bromine Equation.
From www.youtube.com
How to Balance KBr + Cl2 = KCl + Br2 (Potassium bromide + Chlorine gas Chlorine + Bromine Equation Cl + br = clbr is a synthesis reaction where one mole of chlorine [cl] and one mole of bromine [br]. Chlorine + bromine = bromine monochloride. When chloromethane (or methylchloride) reacts with cl 2, another hydrogen is replaced by chlorine atom to give. Cl 2 (aq) + 2ki (aq) → 2kcl (aq) + i 2 (aq) the reaction mixture. Chlorine + Bromine Equation.
From www.numerade.com
SOLVEDThe first preparation of chlorine was similar to the synthesis Chlorine + Bromine Equation When chlorine (as a gas or dissolved in water) is added to sodium bromide solution, the chlorine takes the place of the. When chloromethane (or methylchloride) reacts with cl 2, another hydrogen is replaced by chlorine atom to give. Chlorine + potassium bromide → bromine + potassium chloride symbol equation: Chlorine + bromine = bromine monochloride. In the displacement reactions. Chlorine + Bromine Equation.
From slideplayer.com
Chemical Equations Chapter ppt download Chlorine + Bromine Equation Chlorine + bromine = bromine monochloride. When chlorine (as a gas or dissolved in water) is added to sodium bromide solution, the chlorine takes the place of the. Cl + br = clbr is a synthesis reaction where one mole of chlorine [cl] and one mole of bromine [br]. Cl 2 (g) + 2kbr(aq) →. When chloromethane (or methylchloride) reacts. Chlorine + Bromine Equation.
From kpu.pressbooks.pub
10.4 Reactions of Alkenes Addition of Bromine and Chlorine to Alkenes Chlorine + Bromine Equation Chlorine + bromine = bromine monochloride. Cl 2 (aq) + 2kbr (aq) → 2kcl (aq) + br 2. Chlorine will displace bromine or iodine from an aqueous solution of the metal halide: Cl 2 (g) + 2kbr(aq) →. When chloromethane (or methylchloride) reacts with cl 2, another hydrogen is replaced by chlorine atom to give. Cl 2 (aq) + 2ki. Chlorine + Bromine Equation.
From www.chegg.com
Solved 9. For the reaction of sodium bromide with chlorine Chlorine + Bromine Equation When chloromethane (or methylchloride) reacts with cl 2, another hydrogen is replaced by chlorine atom to give. Cl 2 (g) + 2kbr(aq) →. Cl + br = clbr is a synthesis reaction where one mole of chlorine [cl] and one mole of bromine [br]. Cl 2 (aq) + 2kbr (aq) → 2kcl (aq) + br 2. Cl 2 (aq) +. Chlorine + Bromine Equation.
From www.masterorganicchemistry.com
Selectivity in Free Radical Reactions Bromine vs. Chlorine — Master Chlorine + Bromine Equation Chlorine will displace bromine or iodine from an aqueous solution of the metal halide: Chlorine + potassium bromide → bromine + potassium chloride symbol equation: In the displacement reactions chlorine displaces both bromine and iodine from their compounds and bromine displaces iodine. When chloromethane (or methylchloride) reacts with cl 2, another hydrogen is replaced by chlorine atom to give. Cl. Chlorine + Bromine Equation.
From www.coursehero.com
[Solved] a.) Chlorine and Bromine are both diatomic. Explain the Chlorine + Bromine Equation Cl 2 (aq) + 2ki(aq) → i 2 (aq) +. In the displacement reactions chlorine displaces both bromine and iodine from their compounds and bromine displaces iodine. Cl + br = clbr is a synthesis reaction where one mole of chlorine [cl] and one mole of bromine [br]. Chlorine will displace bromine or iodine from an aqueous solution of the. Chlorine + Bromine Equation.
From www.numerade.com
SOLVEDGaseous chlorine will displace bromide ion from an aqueous Chlorine + Bromine Equation When chlorine (as a gas or dissolved in water) is added to sodium bromide solution, the chlorine takes the place of the. Cl 2 (aq) + 2ki (aq) → 2kcl (aq) + i 2 (aq) the reaction mixture turns darker and iodine solution forms. Chlorine will displace bromine or iodine from an aqueous solution of the metal halide: Cl +. Chlorine + Bromine Equation.
From www.chegg.com
Solved Part A Write a balanced equation for the reaction of Chlorine + Bromine Equation Cl 2 (g) + 2kbr(aq) →. In the displacement reactions chlorine displaces both bromine and iodine from their compounds and bromine displaces iodine. Cl 2 (aq) + 2kbr (aq) → 2kcl (aq) + br 2. When chlorine (as a gas or dissolved in water) is added to sodium bromide solution, the chlorine takes the place of the. Cl + br. Chlorine + Bromine Equation.
From www.youtube.com
Chlorine And Sodium Bromide Make Sodium Chloride And Bromine YouTube Chlorine + Bromine Equation When chloromethane (or methylchloride) reacts with cl 2, another hydrogen is replaced by chlorine atom to give. In the displacement reactions chlorine displaces both bromine and iodine from their compounds and bromine displaces iodine. Cl 2 (aq) + 2ki(aq) → i 2 (aq) +. Cl + br = clbr is a synthesis reaction where one mole of chlorine [cl] and. Chlorine + Bromine Equation.
From www.numerade.com
SOLVEDBromine can be prepared by adding chlorine to an aqueous Chlorine + Bromine Equation Chlorine + bromine = bromine monochloride. Cl 2 (aq) + 2kbr (aq) → 2kcl (aq) + br 2. Chlorine will displace bromine or iodine from an aqueous solution of the metal halide: Chlorine + potassium bromide → bromine + potassium chloride symbol equation: Cl 2 (aq) + 2ki (aq) → 2kcl (aq) + i 2 (aq) the reaction mixture turns. Chlorine + Bromine Equation.
From ar.inspiredpencil.com
Bromine Liquid Formula Chlorine + Bromine Equation Cl 2 (g) + 2kbr(aq) →. Cl 2 (aq) + 2kbr (aq) → 2kcl (aq) + br 2. When chlorine (as a gas or dissolved in water) is added to sodium bromide solution, the chlorine takes the place of the. Cl 2 (aq) + 2ki(aq) → i 2 (aq) +. Chlorine + potassium bromide → bromine + potassium chloride symbol. Chlorine + Bromine Equation.
From www.chegg.com
Solved Chlorine and bromine react in the dark with alkenes. Chlorine + Bromine Equation Chlorine will displace bromine or iodine from an aqueous solution of the metal halide: When chloromethane (or methylchloride) reacts with cl 2, another hydrogen is replaced by chlorine atom to give. Cl 2 (aq) + 2ki(aq) → i 2 (aq) +. Chlorine + potassium bromide → bromine + potassium chloride symbol equation: Cl 2 (aq) + 2kbr (aq) → 2kcl. Chlorine + Bromine Equation.
From www.researchgate.net
(PDF) Equation of state of solid chlorine and bromine Chlorine + Bromine Equation In the displacement reactions chlorine displaces both bromine and iodine from their compounds and bromine displaces iodine. Cl 2 (aq) + 2ki (aq) → 2kcl (aq) + i 2 (aq) the reaction mixture turns darker and iodine solution forms. Chlorine + potassium bromide → bromine + potassium chloride symbol equation: When chlorine (as a gas or dissolved in water) is. Chlorine + Bromine Equation.
From www.numerade.com
SOLVED Part A Enter a balanced equation for the reaction of chlorine Chlorine + Bromine Equation Cl 2 (aq) + 2kbr (aq) → 2kcl (aq) + br 2. Chlorine will displace bromine or iodine from an aqueous solution of the metal halide: Cl 2 (aq) + 2ki(aq) → i 2 (aq) +. Cl + br = clbr is a synthesis reaction where one mole of chlorine [cl] and one mole of bromine [br]. When chloromethane (or. Chlorine + Bromine Equation.
From www.numerade.com
SOLVED Chlorine and bromine react In the dark WIth alkenes The Chlorine + Bromine Equation Cl 2 (aq) + 2kbr (aq) → 2kcl (aq) + br 2. Cl 2 (g) + 2kbr(aq) →. When chlorine (as a gas or dissolved in water) is added to sodium bromide solution, the chlorine takes the place of the. In the displacement reactions chlorine displaces both bromine and iodine from their compounds and bromine displaces iodine. Cl + br. Chlorine + Bromine Equation.
From socratic.org
What is the balanced chemical equation for this reaction Gaseous Chlorine + Bromine Equation When chloromethane (or methylchloride) reacts with cl 2, another hydrogen is replaced by chlorine atom to give. Cl 2 (aq) + 2ki (aq) → 2kcl (aq) + i 2 (aq) the reaction mixture turns darker and iodine solution forms. Cl + br = clbr is a synthesis reaction where one mole of chlorine [cl] and one mole of bromine [br].. Chlorine + Bromine Equation.
From youtube.com
10.2.3 Describe using equations the reactions of methane/ethane with Chlorine + Bromine Equation Chlorine will displace bromine or iodine from an aqueous solution of the metal halide: Cl 2 (aq) + 2kbr (aq) → 2kcl (aq) + br 2. When chlorine (as a gas or dissolved in water) is added to sodium bromide solution, the chlorine takes the place of the. When chloromethane (or methylchloride) reacts with cl 2, another hydrogen is replaced. Chlorine + Bromine Equation.
From slideplayer.com
Biology Dr. Altstiel Naples American High School ppt download Chlorine + Bromine Equation Cl 2 (aq) + 2ki(aq) → i 2 (aq) +. Chlorine will displace bromine or iodine from an aqueous solution of the metal halide: Cl + br = clbr is a synthesis reaction where one mole of chlorine [cl] and one mole of bromine [br]. When chlorine (as a gas or dissolved in water) is added to sodium bromide solution,. Chlorine + Bromine Equation.
From www.numerade.com
SOLVED Sodium chloride reacts with bromine gas, mathrm{Br}_{2}, to Chlorine + Bromine Equation Cl 2 (aq) + 2ki (aq) → 2kcl (aq) + i 2 (aq) the reaction mixture turns darker and iodine solution forms. Chlorine will displace bromine or iodine from an aqueous solution of the metal halide: Chlorine + potassium bromide → bromine + potassium chloride symbol equation: When chloromethane (or methylchloride) reacts with cl 2, another hydrogen is replaced by. Chlorine + Bromine Equation.
From www.numerade.com
SOLVED 1.Chlorine bromine and iodine all react with alkenes such as Chlorine + Bromine Equation Cl 2 (g) + 2kbr(aq) →. Chlorine + bromine = bromine monochloride. Chlorine will displace bromine or iodine from an aqueous solution of the metal halide: When chlorine (as a gas or dissolved in water) is added to sodium bromide solution, the chlorine takes the place of the. Cl 2 (aq) + 2ki (aq) → 2kcl (aq) + i 2. Chlorine + Bromine Equation.
From gcse-revision-chemistry.blogspot.com
Beccy's Chemistry Revision 2018 Section 2 c) Summary Chlorine + Bromine Equation Cl 2 (aq) + 2ki(aq) → i 2 (aq) +. Cl 2 (aq) + 2kbr (aq) → 2kcl (aq) + br 2. Chlorine + bromine = bromine monochloride. When chloromethane (or methylchloride) reacts with cl 2, another hydrogen is replaced by chlorine atom to give. Cl 2 (aq) + 2ki (aq) → 2kcl (aq) + i 2 (aq) the reaction. Chlorine + Bromine Equation.
From www.youtube.com
Type of Reaction for NaBr + Cl2 = NaCl + Br2 YouTube Chlorine + Bromine Equation Chlorine + potassium bromide → bromine + potassium chloride symbol equation: Cl 2 (aq) + 2kbr (aq) → 2kcl (aq) + br 2. Cl 2 (g) + 2kbr(aq) →. Chlorine + bromine = bromine monochloride. Chlorine will displace bromine or iodine from an aqueous solution of the metal halide: Cl + br = clbr is a synthesis reaction where one. Chlorine + Bromine Equation.
From brainly.in
Chlorine, Bromine and Iodine form aDobereiner's triad. the atomic Chlorine + Bromine Equation Cl + br = clbr is a synthesis reaction where one mole of chlorine [cl] and one mole of bromine [br]. When chloromethane (or methylchloride) reacts with cl 2, another hydrogen is replaced by chlorine atom to give. Cl 2 (g) + 2kbr(aq) →. In the displacement reactions chlorine displaces both bromine and iodine from their compounds and bromine displaces. Chlorine + Bromine Equation.
From askanydifference.com
Bromine vs Chlorine Difference and Comparison Chlorine + Bromine Equation When chloromethane (or methylchloride) reacts with cl 2, another hydrogen is replaced by chlorine atom to give. Chlorine + bromine = bromine monochloride. Cl 2 (aq) + 2ki(aq) → i 2 (aq) +. Cl 2 (aq) + 2kbr (aq) → 2kcl (aq) + br 2. In the displacement reactions chlorine displaces both bromine and iodine from their compounds and bromine. Chlorine + Bromine Equation.
From www.researchgate.net
Analytical scheme for radiochemical purification of chlorine, bromine Chlorine + Bromine Equation In the displacement reactions chlorine displaces both bromine and iodine from their compounds and bromine displaces iodine. Cl + br = clbr is a synthesis reaction where one mole of chlorine [cl] and one mole of bromine [br]. Cl 2 (aq) + 2ki(aq) → i 2 (aq) +. Cl 2 (aq) + 2kbr (aq) → 2kcl (aq) + br 2.. Chlorine + Bromine Equation.