Rate Constant K And Temperature . The rate constant k is independent of the concentration of a, b, or c, but it does vary with temperature and surface area. K =ae −e a rt both a and e a are specific to a given reaction.! If the rate constant doubles, for example,. K is the rate constant! Increasing the temperature from 200 k to 350 k causes the rate constant for this particular reaction to increase by a factor of more than 10, whereas the increase in the frequency. It is, however, important to. If the rate constant for a reaction is measure at two temperatures, the activation energy can be determined by taking the ratio. Rate = k [a] where k is the rate constant, and it is the proportionality linkage between the rate and the concentration. E a is the activation energy!
from www.doubtnut.com
If the rate constant for a reaction is measure at two temperatures, the activation energy can be determined by taking the ratio. Rate = k [a] where k is the rate constant, and it is the proportionality linkage between the rate and the concentration. It is, however, important to. The rate constant k is independent of the concentration of a, b, or c, but it does vary with temperature and surface area. Increasing the temperature from 200 k to 350 k causes the rate constant for this particular reaction to increase by a factor of more than 10, whereas the increase in the frequency. E a is the activation energy! K is the rate constant! If the rate constant doubles, for example,. K =ae −e a rt both a and e a are specific to a given reaction.!
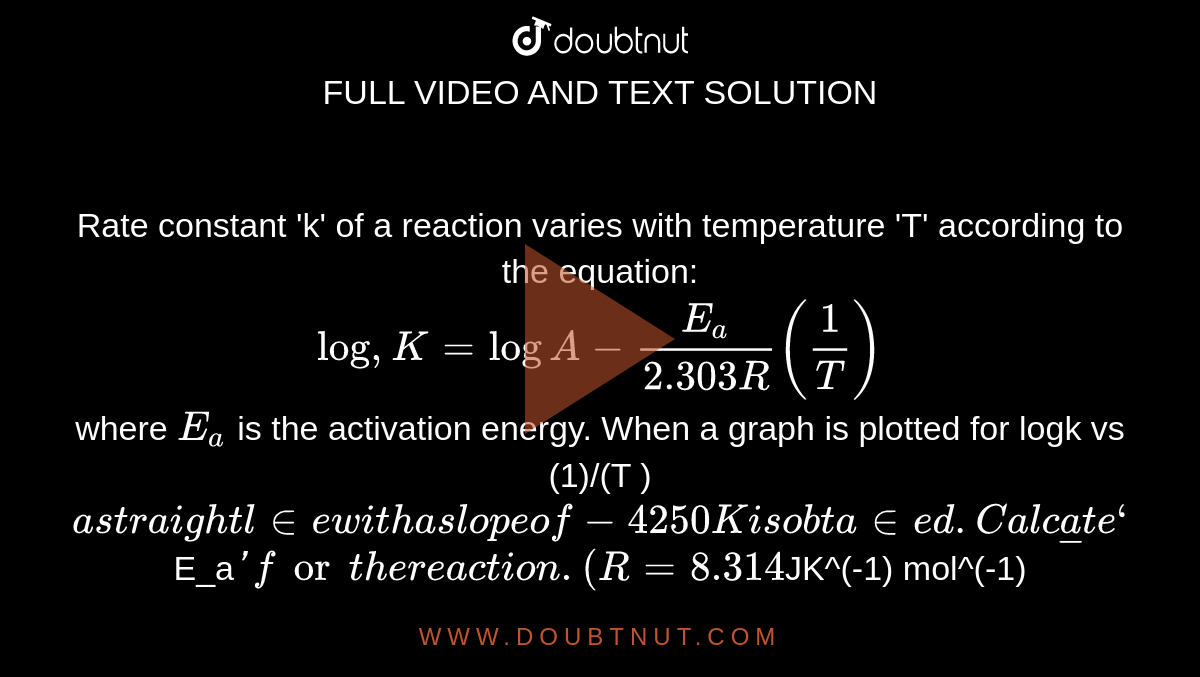
Rate constant 'k' of a reaction varies with temperature 'T' according
Rate Constant K And Temperature If the rate constant for a reaction is measure at two temperatures, the activation energy can be determined by taking the ratio. E a is the activation energy! It is, however, important to. If the rate constant for a reaction is measure at two temperatures, the activation energy can be determined by taking the ratio. If the rate constant doubles, for example,. K is the rate constant! Increasing the temperature from 200 k to 350 k causes the rate constant for this particular reaction to increase by a factor of more than 10, whereas the increase in the frequency. K =ae −e a rt both a and e a are specific to a given reaction.! Rate = k [a] where k is the rate constant, and it is the proportionality linkage between the rate and the concentration. The rate constant k is independent of the concentration of a, b, or c, but it does vary with temperature and surface area.
From askfilo.com
4. Which one of the following given graphs represents the variation of ra.. Rate Constant K And Temperature Increasing the temperature from 200 k to 350 k causes the rate constant for this particular reaction to increase by a factor of more than 10, whereas the increase in the frequency. If the rate constant doubles, for example,. It is, however, important to. K is the rate constant! Rate = k [a] where k is the rate constant, and. Rate Constant K And Temperature.
From www.doubtnut.com
Rate constant 'k' of a reaction varies with temperature 'T' according Rate Constant K And Temperature K is the rate constant! Rate = k [a] where k is the rate constant, and it is the proportionality linkage between the rate and the concentration. It is, however, important to. E a is the activation energy! Increasing the temperature from 200 k to 350 k causes the rate constant for this particular reaction to increase by a factor. Rate Constant K And Temperature.
From www.numerade.com
SOLVED Which one of the following given graphs represents the Rate Constant K And Temperature Rate = k [a] where k is the rate constant, and it is the proportionality linkage between the rate and the concentration. If the rate constant doubles, for example,. K =ae −e a rt both a and e a are specific to a given reaction.! K is the rate constant! If the rate constant for a reaction is measure at. Rate Constant K And Temperature.
From www.youtube.com
16.2 Effect of temperature on the rate constant k (HL) YouTube Rate Constant K And Temperature It is, however, important to. E a is the activation energy! K =ae −e a rt both a and e a are specific to a given reaction.! If the rate constant for a reaction is measure at two temperatures, the activation energy can be determined by taking the ratio. Rate = k [a] where k is the rate constant, and. Rate Constant K And Temperature.
From www.researchgate.net
Relationship between the leaching rate constant k and temperature T Rate Constant K And Temperature Increasing the temperature from 200 k to 350 k causes the rate constant for this particular reaction to increase by a factor of more than 10, whereas the increase in the frequency. Rate = k [a] where k is the rate constant, and it is the proportionality linkage between the rate and the concentration. K =ae −e a rt both. Rate Constant K And Temperature.
From www.youtube.com
Determine the rate constant (k) for a reaction YouTube Rate Constant K And Temperature K =ae −e a rt both a and e a are specific to a given reaction.! It is, however, important to. If the rate constant for a reaction is measure at two temperatures, the activation energy can be determined by taking the ratio. The rate constant k is independent of the concentration of a, b, or c, but it does. Rate Constant K And Temperature.
From www.youtube.com
For a reaction, the values of rate constant k at two Rate Constant K And Temperature If the rate constant doubles, for example,. It is, however, important to. Increasing the temperature from 200 k to 350 k causes the rate constant for this particular reaction to increase by a factor of more than 10, whereas the increase in the frequency. If the rate constant for a reaction is measure at two temperatures, the activation energy can. Rate Constant K And Temperature.
From www.doubtnut.com
Plots showing the variation of the rate constant (k) with temperature Rate Constant K And Temperature Increasing the temperature from 200 k to 350 k causes the rate constant for this particular reaction to increase by a factor of more than 10, whereas the increase in the frequency. It is, however, important to. K =ae −e a rt both a and e a are specific to a given reaction.! The rate constant k is independent of. Rate Constant K And Temperature.
From www.researchgate.net
Variation of the rate constant k with temperature (a,b). • Chicory Rate Constant K And Temperature E a is the activation energy! It is, however, important to. If the rate constant doubles, for example,. K is the rate constant! Rate = k [a] where k is the rate constant, and it is the proportionality linkage between the rate and the concentration. Increasing the temperature from 200 k to 350 k causes the rate constant for this. Rate Constant K And Temperature.
From www.researchgate.net
Relationship between the leaching rate constant k and temperature T Rate Constant K And Temperature Rate = k [a] where k is the rate constant, and it is the proportionality linkage between the rate and the concentration. E a is the activation energy! K is the rate constant! If the rate constant for a reaction is measure at two temperatures, the activation energy can be determined by taking the ratio. It is, however, important to.. Rate Constant K And Temperature.
From www.solvedlib.com
Calculate three values for the rate constant; k, at r… SolvedLib Rate Constant K And Temperature If the rate constant doubles, for example,. If the rate constant for a reaction is measure at two temperatures, the activation energy can be determined by taking the ratio. It is, however, important to. E a is the activation energy! The rate constant k is independent of the concentration of a, b, or c, but it does vary with temperature. Rate Constant K And Temperature.
From www.youtube.com
16.2 Effect of temperature on the rate constant k (HL) YouTube Rate Constant K And Temperature K =ae −e a rt both a and e a are specific to a given reaction.! K is the rate constant! Increasing the temperature from 200 k to 350 k causes the rate constant for this particular reaction to increase by a factor of more than 10, whereas the increase in the frequency. Rate = k [a] where k is. Rate Constant K And Temperature.
From byjus.com
What will be the slope if we plot a graph between rate costant k and Rate Constant K And Temperature The rate constant k is independent of the concentration of a, b, or c, but it does vary with temperature and surface area. Rate = k [a] where k is the rate constant, and it is the proportionality linkage between the rate and the concentration. If the rate constant for a reaction is measure at two temperatures, the activation energy. Rate Constant K And Temperature.
From www.researchgate.net
Reaction Rate dα/dt and Arrhenius Rate Constant k versus Temperature Rate Constant K And Temperature If the rate constant for a reaction is measure at two temperatures, the activation energy can be determined by taking the ratio. The rate constant k is independent of the concentration of a, b, or c, but it does vary with temperature and surface area. E a is the activation energy! Increasing the temperature from 200 k to 350 k. Rate Constant K And Temperature.
From www.chegg.com
Solved The temperature dependence of the reaction rate Rate Constant K And Temperature If the rate constant doubles, for example,. E a is the activation energy! K is the rate constant! The rate constant k is independent of the concentration of a, b, or c, but it does vary with temperature and surface area. Increasing the temperature from 200 k to 350 k causes the rate constant for this particular reaction to increase. Rate Constant K And Temperature.
From www.slideserve.com
PPT Rate Laws PowerPoint Presentation, free download ID4905523 Rate Constant K And Temperature It is, however, important to. K is the rate constant! Increasing the temperature from 200 k to 350 k causes the rate constant for this particular reaction to increase by a factor of more than 10, whereas the increase in the frequency. If the rate constant doubles, for example,. If the rate constant for a reaction is measure at two. Rate Constant K And Temperature.
From www.slideshare.net
Nyb F09 Unit 2 Slides 26 57 Rate Constant K And Temperature If the rate constant doubles, for example,. The rate constant k is independent of the concentration of a, b, or c, but it does vary with temperature and surface area. E a is the activation energy! Increasing the temperature from 200 k to 350 k causes the rate constant for this particular reaction to increase by a factor of more. Rate Constant K And Temperature.
From www.chegg.com
Solved The rate constant k for a certain reaction is Rate Constant K And Temperature K =ae −e a rt both a and e a are specific to a given reaction.! If the rate constant for a reaction is measure at two temperatures, the activation energy can be determined by taking the ratio. It is, however, important to. If the rate constant doubles, for example,. Rate = k [a] where k is the rate constant,. Rate Constant K And Temperature.
From www.slideserve.com
PPT Chapter 16 Rates and Mechanisms of Chemical Reactions Rate Constant K And Temperature K =ae −e a rt both a and e a are specific to a given reaction.! E a is the activation energy! The rate constant k is independent of the concentration of a, b, or c, but it does vary with temperature and surface area. Rate = k [a] where k is the rate constant, and it is the proportionality. Rate Constant K And Temperature.
From www.chegg.com
Solved The Arrhenius equation shows the relationship between Rate Constant K And Temperature If the rate constant for a reaction is measure at two temperatures, the activation energy can be determined by taking the ratio. If the rate constant doubles, for example,. It is, however, important to. Rate = k [a] where k is the rate constant, and it is the proportionality linkage between the rate and the concentration. Increasing the temperature from. Rate Constant K And Temperature.
From www.toppr.com
Plots showing the variation of rate constant K with temperature (T) are Rate Constant K And Temperature K is the rate constant! E a is the activation energy! K =ae −e a rt both a and e a are specific to a given reaction.! It is, however, important to. If the rate constant doubles, for example,. Rate = k [a] where k is the rate constant, and it is the proportionality linkage between the rate and the. Rate Constant K And Temperature.
From www.youtube.com
Temperature and Arrhenius equation YouTube Rate Constant K And Temperature If the rate constant for a reaction is measure at two temperatures, the activation energy can be determined by taking the ratio. It is, however, important to. Rate = k [a] where k is the rate constant, and it is the proportionality linkage between the rate and the concentration. K is the rate constant! If the rate constant doubles, for. Rate Constant K And Temperature.
From www.youtube.com
How To Determine The Units Of The Rate Constant K Chemical Rate Constant K And Temperature K =ae −e a rt both a and e a are specific to a given reaction.! E a is the activation energy! If the rate constant for a reaction is measure at two temperatures, the activation energy can be determined by taking the ratio. If the rate constant doubles, for example,. It is, however, important to. The rate constant k. Rate Constant K And Temperature.
From www.youtube.com
CHEM 201 Temperature Dependence of Rate Constant YouTube Rate Constant K And Temperature The rate constant k is independent of the concentration of a, b, or c, but it does vary with temperature and surface area. K is the rate constant! E a is the activation energy! It is, however, important to. If the rate constant for a reaction is measure at two temperatures, the activation energy can be determined by taking the. Rate Constant K And Temperature.
From www.slideserve.com
PPT Chemical Equilibrium PowerPoint Presentation, free download ID Rate Constant K And Temperature If the rate constant for a reaction is measure at two temperatures, the activation energy can be determined by taking the ratio. Increasing the temperature from 200 k to 350 k causes the rate constant for this particular reaction to increase by a factor of more than 10, whereas the increase in the frequency. K is the rate constant! Rate. Rate Constant K And Temperature.
From www.numerade.com
SOLVEDThe rate constant (k) for a reaction was measured as a function Rate Constant K And Temperature The rate constant k is independent of the concentration of a, b, or c, but it does vary with temperature and surface area. Increasing the temperature from 200 k to 350 k causes the rate constant for this particular reaction to increase by a factor of more than 10, whereas the increase in the frequency. If the rate constant for. Rate Constant K And Temperature.
From www.researchgate.net
Temperature dependence of the first order rate constant K in Download Rate Constant K And Temperature K =ae −e a rt both a and e a are specific to a given reaction.! Rate = k [a] where k is the rate constant, and it is the proportionality linkage between the rate and the concentration. E a is the activation energy! K is the rate constant! Increasing the temperature from 200 k to 350 k causes the. Rate Constant K And Temperature.
From plot.ly
Rate constant in function of temperature scatter chart made by Ayoub Rate Constant K And Temperature It is, however, important to. If the rate constant doubles, for example,. Rate = k [a] where k is the rate constant, and it is the proportionality linkage between the rate and the concentration. E a is the activation energy! K =ae −e a rt both a and e a are specific to a given reaction.! K is the rate. Rate Constant K And Temperature.
From www.doubtnut.com
Rate constant vs temperature graph looks like If the activation energ Rate Constant K And Temperature E a is the activation energy! If the rate constant for a reaction is measure at two temperatures, the activation energy can be determined by taking the ratio. Rate = k [a] where k is the rate constant, and it is the proportionality linkage between the rate and the concentration. If the rate constant doubles, for example,. The rate constant. Rate Constant K And Temperature.
From kunduz.com
[ANSWERED] Rate constant K varies with temperature as given by equation Rate Constant K And Temperature If the rate constant for a reaction is measure at two temperatures, the activation energy can be determined by taking the ratio. E a is the activation energy! Increasing the temperature from 200 k to 350 k causes the rate constant for this particular reaction to increase by a factor of more than 10, whereas the increase in the frequency.. Rate Constant K And Temperature.
From www.doubtnut.com
Plots showing the variation of the rate constant (k) with temperature Rate Constant K And Temperature Increasing the temperature from 200 k to 350 k causes the rate constant for this particular reaction to increase by a factor of more than 10, whereas the increase in the frequency. K =ae −e a rt both a and e a are specific to a given reaction.! Rate = k [a] where k is the rate constant, and it. Rate Constant K And Temperature.
From www.savemyexams.com
The Rate Constant (HL) HL IB Chemistry Revision Notes 2025 Save My Rate Constant K And Temperature It is, however, important to. Increasing the temperature from 200 k to 350 k causes the rate constant for this particular reaction to increase by a factor of more than 10, whereas the increase in the frequency. If the rate constant for a reaction is measure at two temperatures, the activation energy can be determined by taking the ratio. K. Rate Constant K And Temperature.
From haipernews.com
How To Calculate Equilibrium Constant K Haiper Rate Constant K And Temperature Rate = k [a] where k is the rate constant, and it is the proportionality linkage between the rate and the concentration. E a is the activation energy! If the rate constant for a reaction is measure at two temperatures, the activation energy can be determined by taking the ratio. K =ae −e a rt both a and e a. Rate Constant K And Temperature.
From www.youtube.com
16.3.1 Describe qualitatively the relationship between the rate Rate Constant K And Temperature E a is the activation energy! It is, however, important to. If the rate constant for a reaction is measure at two temperatures, the activation energy can be determined by taking the ratio. If the rate constant doubles, for example,. K is the rate constant! Increasing the temperature from 200 k to 350 k causes the rate constant for this. Rate Constant K And Temperature.
From fin3tutor.blogspot.com
How To Calculate Rate Constant With Temperature Rate Constant K And Temperature It is, however, important to. K =ae −e a rt both a and e a are specific to a given reaction.! Rate = k [a] where k is the rate constant, and it is the proportionality linkage between the rate and the concentration. E a is the activation energy! If the rate constant doubles, for example,. The rate constant k. Rate Constant K And Temperature.