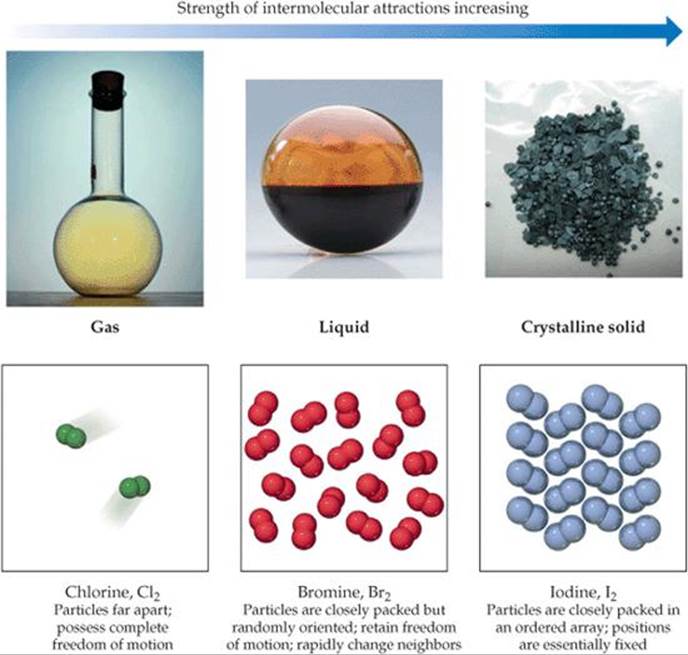
Intermolecular Forces Of Bromine In Water . Identify the types of intermolecular forces experienced by specific molecules based on their structures; • when a substance melts or boils, intermolecular forces are broken. By the end of this section, you will be able to: Understand the types of intermolecular forces: The properties of liquids are intermediate between those of gases and solids. It is unlikely to be a solid at room temperature. • intermolecular forces are much weaker than ionic or covalent bonds. Thus, diatomic bromine does not have any intermolecular forces other than dispersion forces. To describe the intermolecular forces in liquids. Describe the types of intermolecular forces possible between atoms or molecules in condensed phases (dispersion. Two liquids, such as bromine and water, that are of moderate mutual solubility are said to be partially miscible. Explain the relation between the.
from schoolbag.info
Two liquids, such as bromine and water, that are of moderate mutual solubility are said to be partially miscible. Describe the types of intermolecular forces possible between atoms or molecules in condensed phases (dispersion. • intermolecular forces are much weaker than ionic or covalent bonds. Identify the types of intermolecular forces experienced by specific molecules based on their structures; Thus, diatomic bromine does not have any intermolecular forces other than dispersion forces. • when a substance melts or boils, intermolecular forces are broken. The properties of liquids are intermediate between those of gases and solids. It is unlikely to be a solid at room temperature. By the end of this section, you will be able to: Understand the types of intermolecular forces:
For a given substance, do you expect the density of the substance in its liquid state to be
Intermolecular Forces Of Bromine In Water By the end of this section, you will be able to: Explain the relation between the. Describe the types of intermolecular forces possible between atoms or molecules in condensed phases (dispersion. The properties of liquids are intermediate between those of gases and solids. • when a substance melts or boils, intermolecular forces are broken. Identify the types of intermolecular forces experienced by specific molecules based on their structures; It is unlikely to be a solid at room temperature. To describe the intermolecular forces in liquids. • intermolecular forces are much weaker than ionic or covalent bonds. Thus, diatomic bromine does not have any intermolecular forces other than dispersion forces. By the end of this section, you will be able to: Understand the types of intermolecular forces: Two liquids, such as bromine and water, that are of moderate mutual solubility are said to be partially miscible.
From manuallistcantabank.z21.web.core.windows.net
Lewis Dot Diagram For Bromine Intermolecular Forces Of Bromine In Water Understand the types of intermolecular forces: By the end of this section, you will be able to: Explain the relation between the. Identify the types of intermolecular forces experienced by specific molecules based on their structures; It is unlikely to be a solid at room temperature. To describe the intermolecular forces in liquids. Thus, diatomic bromine does not have any. Intermolecular Forces Of Bromine In Water.
From www.peoi.org
Chapter 10 Section A Intermolecular Forces Intermolecular Forces Of Bromine In Water • when a substance melts or boils, intermolecular forces are broken. By the end of this section, you will be able to: Two liquids, such as bromine and water, that are of moderate mutual solubility are said to be partially miscible. Identify the types of intermolecular forces experienced by specific molecules based on their structures; Explain the relation between the.. Intermolecular Forces Of Bromine In Water.
From www.bartleby.com
Answered Which intermolecular forces do the… bartleby Intermolecular Forces Of Bromine In Water Thus, diatomic bromine does not have any intermolecular forces other than dispersion forces. Identify the types of intermolecular forces experienced by specific molecules based on their structures; Two liquids, such as bromine and water, that are of moderate mutual solubility are said to be partially miscible. Describe the types of intermolecular forces possible between atoms or molecules in condensed phases. Intermolecular Forces Of Bromine In Water.
From www.numerade.com
SOLVED Use molecular structure and intermolecular bonding to describe why bromine has a lower Intermolecular Forces Of Bromine In Water Identify the types of intermolecular forces experienced by specific molecules based on their structures; • when a substance melts or boils, intermolecular forces are broken. It is unlikely to be a solid at room temperature. To describe the intermolecular forces in liquids. Understand the types of intermolecular forces: • intermolecular forces are much weaker than ionic or covalent bonds. Describe. Intermolecular Forces Of Bromine In Water.
From slideplayer.com
Intermolecular Forces ppt download Intermolecular Forces Of Bromine In Water Thus, diatomic bromine does not have any intermolecular forces other than dispersion forces. It is unlikely to be a solid at room temperature. Describe the types of intermolecular forces possible between atoms or molecules in condensed phases (dispersion. To describe the intermolecular forces in liquids. • when a substance melts or boils, intermolecular forces are broken. The properties of liquids. Intermolecular Forces Of Bromine In Water.
From www.numerade.com
SOLVED Which answer best describes the strongest intermolecular force that exists between two Intermolecular Forces Of Bromine In Water The properties of liquids are intermediate between those of gases and solids. Two liquids, such as bromine and water, that are of moderate mutual solubility are said to be partially miscible. Explain the relation between the. Thus, diatomic bromine does not have any intermolecular forces other than dispersion forces. Understand the types of intermolecular forces: By the end of this. Intermolecular Forces Of Bromine In Water.
From www.chegg.com
Solved Which intermolecular forces do the particles in the Intermolecular Forces Of Bromine In Water Identify the types of intermolecular forces experienced by specific molecules based on their structures; • intermolecular forces are much weaker than ionic or covalent bonds. To describe the intermolecular forces in liquids. By the end of this section, you will be able to: Two liquids, such as bromine and water, that are of moderate mutual solubility are said to be. Intermolecular Forces Of Bromine In Water.
From www.chegg.com
Solved Which intermolecular forces do the particles in the Intermolecular Forces Of Bromine In Water It is unlikely to be a solid at room temperature. Two liquids, such as bromine and water, that are of moderate mutual solubility are said to be partially miscible. Explain the relation between the. The properties of liquids are intermediate between those of gases and solids. Describe the types of intermolecular forces possible between atoms or molecules in condensed phases. Intermolecular Forces Of Bromine In Water.
From www.slideserve.com
PPT Liquids, solids, & intermolecular forces PowerPoint Presentation ID1459744 Intermolecular Forces Of Bromine In Water Describe the types of intermolecular forces possible between atoms or molecules in condensed phases (dispersion. • intermolecular forces are much weaker than ionic or covalent bonds. Understand the types of intermolecular forces: Two liquids, such as bromine and water, that are of moderate mutual solubility are said to be partially miscible. To describe the intermolecular forces in liquids. Identify the. Intermolecular Forces Of Bromine In Water.
From www.researchgate.net
(a) Intermolecular bromine/p interaction in the complex; (b) formation... Download Scientific Intermolecular Forces Of Bromine In Water • intermolecular forces are much weaker than ionic or covalent bonds. Explain the relation between the. Identify the types of intermolecular forces experienced by specific molecules based on their structures; Thus, diatomic bromine does not have any intermolecular forces other than dispersion forces. Describe the types of intermolecular forces possible between atoms or molecules in condensed phases (dispersion. Two liquids,. Intermolecular Forces Of Bromine In Water.
From www.youtube.com
BrF Polar or Nonpolar (Bromine fluoride) YouTube Intermolecular Forces Of Bromine In Water Explain the relation between the. To describe the intermolecular forces in liquids. Two liquids, such as bromine and water, that are of moderate mutual solubility are said to be partially miscible. • intermolecular forces are much weaker than ionic or covalent bonds. The properties of liquids are intermediate between those of gases and solids. Describe the types of intermolecular forces. Intermolecular Forces Of Bromine In Water.
From www.chegg.com
Solved Which intermolecular forces do the particle in the Intermolecular Forces Of Bromine In Water To describe the intermolecular forces in liquids. Understand the types of intermolecular forces: Thus, diatomic bromine does not have any intermolecular forces other than dispersion forces. Explain the relation between the. The properties of liquids are intermediate between those of gases and solids. Describe the types of intermolecular forces possible between atoms or molecules in condensed phases (dispersion. • intermolecular. Intermolecular Forces Of Bromine In Water.
From www.numerade.com
SOLVED List the intermolecular forces present in hydrogen halides. Explain why hydrogen Intermolecular Forces Of Bromine In Water The properties of liquids are intermediate between those of gases and solids. Thus, diatomic bromine does not have any intermolecular forces other than dispersion forces. Understand the types of intermolecular forces: By the end of this section, you will be able to: To describe the intermolecular forces in liquids. Identify the types of intermolecular forces experienced by specific molecules based. Intermolecular Forces Of Bromine In Water.
From www.numerade.com
SOLVED Decide which Intermolecular forces act betwcen the molecules of each compound in the Intermolecular Forces Of Bromine In Water Explain the relation between the. The properties of liquids are intermediate between those of gases and solids. To describe the intermolecular forces in liquids. Thus, diatomic bromine does not have any intermolecular forces other than dispersion forces. • when a substance melts or boils, intermolecular forces are broken. Describe the types of intermolecular forces possible between atoms or molecules in. Intermolecular Forces Of Bromine In Water.
From www.youtube.com
Hydrogen Bonds In Water Explained Intermolecular Forces YouTube Intermolecular Forces Of Bromine In Water • when a substance melts or boils, intermolecular forces are broken. To describe the intermolecular forces in liquids. Two liquids, such as bromine and water, that are of moderate mutual solubility are said to be partially miscible. Thus, diatomic bromine does not have any intermolecular forces other than dispersion forces. Describe the types of intermolecular forces possible between atoms or. Intermolecular Forces Of Bromine In Water.
From learningchemistryeasily.blogspot.com
Learning Chemistry Easily Intermolecular Forces In Solutions Intermolecular Forces Of Bromine In Water The properties of liquids are intermediate between those of gases and solids. Understand the types of intermolecular forces: Explain the relation between the. Identify the types of intermolecular forces experienced by specific molecules based on their structures; Describe the types of intermolecular forces possible between atoms or molecules in condensed phases (dispersion. • intermolecular forces are much weaker than ionic. Intermolecular Forces Of Bromine In Water.
From sciencenotes.org
Intermolecular Forces in Chemistry Intermolecular Forces Of Bromine In Water Two liquids, such as bromine and water, that are of moderate mutual solubility are said to be partially miscible. • intermolecular forces are much weaker than ionic or covalent bonds. Describe the types of intermolecular forces possible between atoms or molecules in condensed phases (dispersion. The properties of liquids are intermediate between those of gases and solids. By the end. Intermolecular Forces Of Bromine In Water.
From www.coursehero.com
[Solved] a.) Chlorine and Bromine are both diatomic. Explain the difference... Course Hero Intermolecular Forces Of Bromine In Water Describe the types of intermolecular forces possible between atoms or molecules in condensed phases (dispersion. Thus, diatomic bromine does not have any intermolecular forces other than dispersion forces. Understand the types of intermolecular forces: By the end of this section, you will be able to: To describe the intermolecular forces in liquids. The properties of liquids are intermediate between those. Intermolecular Forces Of Bromine In Water.
From www.numerade.com
In addition to dispersion forces, what intermolecular forces are present in a solution between Intermolecular Forces Of Bromine In Water • when a substance melts or boils, intermolecular forces are broken. Understand the types of intermolecular forces: Identify the types of intermolecular forces experienced by specific molecules based on their structures; It is unlikely to be a solid at room temperature. Describe the types of intermolecular forces possible between atoms or molecules in condensed phases (dispersion. By the end of. Intermolecular Forces Of Bromine In Water.
From slideplayer.com
Intermolecular Forces ppt download Intermolecular Forces Of Bromine In Water To describe the intermolecular forces in liquids. It is unlikely to be a solid at room temperature. Thus, diatomic bromine does not have any intermolecular forces other than dispersion forces. Describe the types of intermolecular forces possible between atoms or molecules in condensed phases (dispersion. Understand the types of intermolecular forces: Explain the relation between the. By the end of. Intermolecular Forces Of Bromine In Water.
From slideplayer.com
Determine the Bond Type (Electronegativities) ppt download Intermolecular Forces Of Bromine In Water • when a substance melts or boils, intermolecular forces are broken. • intermolecular forces are much weaker than ionic or covalent bonds. Thus, diatomic bromine does not have any intermolecular forces other than dispersion forces. It is unlikely to be a solid at room temperature. By the end of this section, you will be able to: Two liquids, such as. Intermolecular Forces Of Bromine In Water.
From courses.lumenlearning.com
Intermolecular Forces Physical Properties of Organic Compounds MCC Organic Chemistry Intermolecular Forces Of Bromine In Water Describe the types of intermolecular forces possible between atoms or molecules in condensed phases (dispersion. Identify the types of intermolecular forces experienced by specific molecules based on their structures; Understand the types of intermolecular forces: Two liquids, such as bromine and water, that are of moderate mutual solubility are said to be partially miscible. Thus, diatomic bromine does not have. Intermolecular Forces Of Bromine In Water.
From fphoto.photoshelter.com
science element bromine Fundamental Photographs The Art of Science Intermolecular Forces Of Bromine In Water By the end of this section, you will be able to: The properties of liquids are intermediate between those of gases and solids. To describe the intermolecular forces in liquids. • when a substance melts or boils, intermolecular forces are broken. It is unlikely to be a solid at room temperature. • intermolecular forces are much weaker than ionic or. Intermolecular Forces Of Bromine In Water.
From www.slideserve.com
PPT Comparing Intermolecular Force’s PowerPoint Presentation, free download ID6047087 Intermolecular Forces Of Bromine In Water The properties of liquids are intermediate between those of gases and solids. • intermolecular forces are much weaker than ionic or covalent bonds. By the end of this section, you will be able to: Identify the types of intermolecular forces experienced by specific molecules based on their structures; Understand the types of intermolecular forces: Describe the types of intermolecular forces. Intermolecular Forces Of Bromine In Water.
From www.numerade.com
SOLVED Decide which intermolecular forces act between the molecules of each compound in the Intermolecular Forces Of Bromine In Water It is unlikely to be a solid at room temperature. Explain the relation between the. • intermolecular forces are much weaker than ionic or covalent bonds. By the end of this section, you will be able to: Thus, diatomic bromine does not have any intermolecular forces other than dispersion forces. To describe the intermolecular forces in liquids. Describe the types. Intermolecular Forces Of Bromine In Water.
From www.chegg.com
Solved Which intermolecular forces do the particles in the Intermolecular Forces Of Bromine In Water Describe the types of intermolecular forces possible between atoms or molecules in condensed phases (dispersion. Identify the types of intermolecular forces experienced by specific molecules based on their structures; To describe the intermolecular forces in liquids. Understand the types of intermolecular forces: It is unlikely to be a solid at room temperature. Explain the relation between the. By the end. Intermolecular Forces Of Bromine In Water.
From www.clutchprep.com
Intermolecular Forces Chemistry Video Clutch Prep Intermolecular Forces Of Bromine In Water To describe the intermolecular forces in liquids. Thus, diatomic bromine does not have any intermolecular forces other than dispersion forces. Identify the types of intermolecular forces experienced by specific molecules based on their structures; • when a substance melts or boils, intermolecular forces are broken. • intermolecular forces are much weaker than ionic or covalent bonds. The properties of liquids. Intermolecular Forces Of Bromine In Water.
From fphoto.photoshelter.com
science element bromine Fundamental Photographs The Art of Science Intermolecular Forces Of Bromine In Water The properties of liquids are intermediate between those of gases and solids. To describe the intermolecular forces in liquids. Describe the types of intermolecular forces possible between atoms or molecules in condensed phases (dispersion. • when a substance melts or boils, intermolecular forces are broken. Explain the relation between the. By the end of this section, you will be able. Intermolecular Forces Of Bromine In Water.
From www.chemistrysteps.com
Boiling Point and Melting Point in Organic Chemistry Chemistry Steps Intermolecular Forces Of Bromine In Water Identify the types of intermolecular forces experienced by specific molecules based on their structures; • when a substance melts or boils, intermolecular forces are broken. By the end of this section, you will be able to: To describe the intermolecular forces in liquids. The properties of liquids are intermediate between those of gases and solids. Thus, diatomic bromine does not. Intermolecular Forces Of Bromine In Water.
From schoolbag.info
For a given substance, do you expect the density of the substance in its liquid state to be Intermolecular Forces Of Bromine In Water • when a substance melts or boils, intermolecular forces are broken. The properties of liquids are intermediate between those of gases and solids. Two liquids, such as bromine and water, that are of moderate mutual solubility are said to be partially miscible. Understand the types of intermolecular forces: It is unlikely to be a solid at room temperature. Thus, diatomic. Intermolecular Forces Of Bromine In Water.
From www.youtube.com
Intermolecular Forces for Br2 (Diatomic Bromine) YouTube Intermolecular Forces Of Bromine In Water It is unlikely to be a solid at room temperature. Identify the types of intermolecular forces experienced by specific molecules based on their structures; Thus, diatomic bromine does not have any intermolecular forces other than dispersion forces. Understand the types of intermolecular forces: Describe the types of intermolecular forces possible between atoms or molecules in condensed phases (dispersion. Explain the. Intermolecular Forces Of Bromine In Water.
From www.numerade.com
SOLVED Decide which intermolecular forces act between the molecules of each compound in the Intermolecular Forces Of Bromine In Water Explain the relation between the. • intermolecular forces are much weaker than ionic or covalent bonds. Understand the types of intermolecular forces: Thus, diatomic bromine does not have any intermolecular forces other than dispersion forces. Two liquids, such as bromine and water, that are of moderate mutual solubility are said to be partially miscible. • when a substance melts or. Intermolecular Forces Of Bromine In Water.
From slideplayer.com
Intermolecular Forces ppt download Intermolecular Forces Of Bromine In Water It is unlikely to be a solid at room temperature. Understand the types of intermolecular forces: Two liquids, such as bromine and water, that are of moderate mutual solubility are said to be partially miscible. Thus, diatomic bromine does not have any intermolecular forces other than dispersion forces. Explain the relation between the. The properties of liquids are intermediate between. Intermolecular Forces Of Bromine In Water.
From www.coursehero.com
[Solved] Intermolecular Forces & Substances. A. Intermolecular Forces 1.... Course Hero Intermolecular Forces Of Bromine In Water By the end of this section, you will be able to: Describe the types of intermolecular forces possible between atoms or molecules in condensed phases (dispersion. The properties of liquids are intermediate between those of gases and solids. Two liquids, such as bromine and water, that are of moderate mutual solubility are said to be partially miscible. • when a. Intermolecular Forces Of Bromine In Water.
From www.numerade.com
SOLVEDAt room temperature, bromine (Br2) is a corrosive red liquid, whereas iodine (I2) is a Intermolecular Forces Of Bromine In Water Thus, diatomic bromine does not have any intermolecular forces other than dispersion forces. • when a substance melts or boils, intermolecular forces are broken. Two liquids, such as bromine and water, that are of moderate mutual solubility are said to be partially miscible. The properties of liquids are intermediate between those of gases and solids. Explain the relation between the.. Intermolecular Forces Of Bromine In Water.