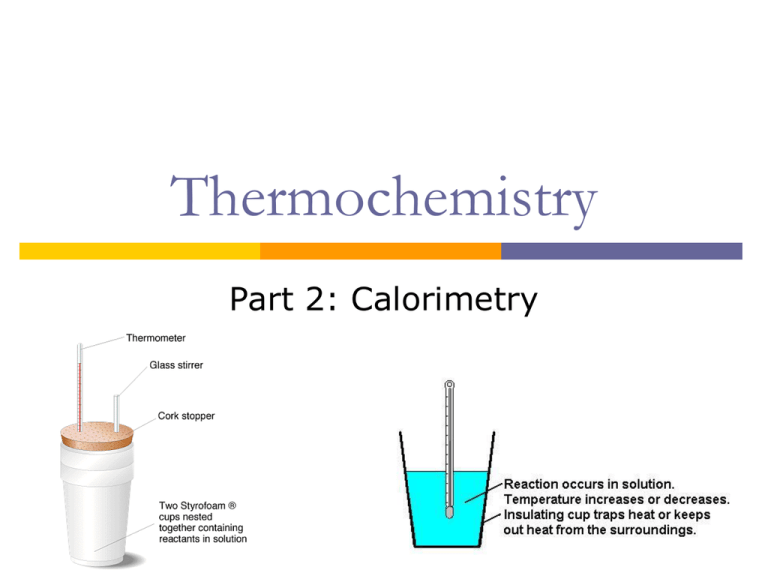
Calorimetry Wikipedia . differential scanning calorimetry (dsc) is a thermoanalytical technique in which the difference in the amount of heat required. a container that prevents heat transfer in or out is called a calorimeter, and the use of a calorimeter to make measurements (typically of heat or specific heat. a calorimeter is a device used for calorimetry, or the process of measuring the heat of chemical reactions or physical. calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat. a calorimeter is a tool that measures the amount of thermal energy contained in a substance, or released in a reaction.
from studylib.net
a container that prevents heat transfer in or out is called a calorimeter, and the use of a calorimeter to make measurements (typically of heat or specific heat. differential scanning calorimetry (dsc) is a thermoanalytical technique in which the difference in the amount of heat required. a calorimeter is a tool that measures the amount of thermal energy contained in a substance, or released in a reaction. a calorimeter is a device used for calorimetry, or the process of measuring the heat of chemical reactions or physical. calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat.
Calorimetry
Calorimetry Wikipedia calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. a container that prevents heat transfer in or out is called a calorimeter, and the use of a calorimeter to make measurements (typically of heat or specific heat. calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat. a calorimeter is a tool that measures the amount of thermal energy contained in a substance, or released in a reaction. a calorimeter is a device used for calorimetry, or the process of measuring the heat of chemical reactions or physical. differential scanning calorimetry (dsc) is a thermoanalytical technique in which the difference in the amount of heat required. calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction.
From studylib.net
Calorimetry Calorimetry Wikipedia a calorimeter is a device used for calorimetry, or the process of measuring the heat of chemical reactions or physical. calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat. differential scanning calorimetry (dsc) is a thermoanalytical technique in which the difference in the amount of heat required.. Calorimetry Wikipedia.
From punchlistzero.com
Is Heat a State Function? Punchlist Zero Calorimetry Wikipedia differential scanning calorimetry (dsc) is a thermoanalytical technique in which the difference in the amount of heat required. calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat. a calorimeter is a device used for calorimetry, or the process of measuring the heat of chemical reactions or physical.. Calorimetry Wikipedia.
From www.science-revision.co.uk
Calorimetry Calorimetry Wikipedia calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat. differential scanning calorimetry (dsc) is a thermoanalytical technique in which the difference in the amount of heat required. a container that prevents heat transfer in or out is called a calorimeter, and the use of a calorimeter to. Calorimetry Wikipedia.
From www.sliderbase.com
Basic Thermochemistry Presentation Chemistry Calorimetry Wikipedia a container that prevents heat transfer in or out is called a calorimeter, and the use of a calorimeter to make measurements (typically of heat or specific heat. calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat. calorimetry is the process of measuring the amount of heat. Calorimetry Wikipedia.
From www.vrogue.co
Differential Scanning Calorimetry Wikipedia vrogue.co Calorimetry Wikipedia calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat. calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. a calorimeter is a tool that measures the amount of thermal energy contained in a substance, or released in a reaction.. Calorimetry Wikipedia.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation, free download ID6133898 Calorimetry Wikipedia calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat. a calorimeter is a device used for calorimetry, or the process of measuring the heat of chemical reactions or physical. calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. . Calorimetry Wikipedia.
From users.highland.edu
Calorimetry Calorimetry Wikipedia calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. a container that prevents heat transfer in or out is called a calorimeter, and the use of a calorimeter to make measurements (typically of heat or specific heat. calorimeter, device for measuring the heat developed during a mechanical, electrical, or. Calorimetry Wikipedia.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation, free download ID6912350 Calorimetry Wikipedia a container that prevents heat transfer in or out is called a calorimeter, and the use of a calorimeter to make measurements (typically of heat or specific heat. calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat. calorimetry is the process of measuring the amount of heat. Calorimetry Wikipedia.
From mechasource.blogspot.com
An Introduction To Calorimetry types And Uses , Bomb and Boy,s Gas Calorimetry Wikipedia differential scanning calorimetry (dsc) is a thermoanalytical technique in which the difference in the amount of heat required. a container that prevents heat transfer in or out is called a calorimeter, and the use of a calorimeter to make measurements (typically of heat or specific heat. a calorimeter is a device used for calorimetry, or the process. Calorimetry Wikipedia.
From slideplayer.com
SPECIFIC HEAT & CALORIMETRY ppt download Calorimetry Wikipedia a calorimeter is a tool that measures the amount of thermal energy contained in a substance, or released in a reaction. calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. differential scanning calorimetry (dsc) is a thermoanalytical technique in which the difference in the amount of heat required. . Calorimetry Wikipedia.
From www.youtube.com
Principle of Calorimetry YouTube Calorimetry Wikipedia a calorimeter is a device used for calorimetry, or the process of measuring the heat of chemical reactions or physical. differential scanning calorimetry (dsc) is a thermoanalytical technique in which the difference in the amount of heat required. a container that prevents heat transfer in or out is called a calorimeter, and the use of a calorimeter. Calorimetry Wikipedia.
From eduinput.com
CalorimeterDefinition, History, Construction, Types, And Uses Calorimetry Wikipedia calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. a container that prevents heat transfer in or out is called a calorimeter, and the use of a calorimeter to make measurements (typically of heat or specific heat. calorimeter, device for measuring the heat developed during a mechanical, electrical, or. Calorimetry Wikipedia.
From www.youtube.com
BASIC PRINCIPLE OF CALORIMETRY YouTube Calorimetry Wikipedia a container that prevents heat transfer in or out is called a calorimeter, and the use of a calorimeter to make measurements (typically of heat or specific heat. a calorimeter is a tool that measures the amount of thermal energy contained in a substance, or released in a reaction. calorimetry is the process of measuring the amount. Calorimetry Wikipedia.
From www.scribd.com
Calorimeter From Wikipedia, The Free Encyclopedia PDF Differential Calorimetry Wikipedia a container that prevents heat transfer in or out is called a calorimeter, and the use of a calorimeter to make measurements (typically of heat or specific heat. differential scanning calorimetry (dsc) is a thermoanalytical technique in which the difference in the amount of heat required. calorimeter, device for measuring the heat developed during a mechanical, electrical,. Calorimetry Wikipedia.
From www.chemistrystudent.com
Calorimetry (ALevel) ChemistryStudent Calorimetry Wikipedia calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. differential scanning calorimetry (dsc) is a thermoanalytical technique in which the difference in the amount of heat required. a container that prevents heat transfer in or out is called a calorimeter, and the use of a calorimeter to make measurements. Calorimetry Wikipedia.
From www.embibe.com
Explain the construction of a calorimeter Draw the necessary figure Calorimetry Wikipedia a calorimeter is a tool that measures the amount of thermal energy contained in a substance, or released in a reaction. calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat. a calorimeter is a device used for calorimetry, or the process of measuring the heat of chemical. Calorimetry Wikipedia.
From www.pinterest.com
ITC1 Isothermal titration calorimetry Wikipedia in 2021 Calorimetry Wikipedia a calorimeter is a tool that measures the amount of thermal energy contained in a substance, or released in a reaction. a container that prevents heat transfer in or out is called a calorimeter, and the use of a calorimeter to make measurements (typically of heat or specific heat. calorimetry is the process of measuring the amount. Calorimetry Wikipedia.
From www.pinterest.com
Thermal transitions in amorphous and semicrystalline polymers Calorimetry Wikipedia differential scanning calorimetry (dsc) is a thermoanalytical technique in which the difference in the amount of heat required. a calorimeter is a tool that measures the amount of thermal energy contained in a substance, or released in a reaction. a container that prevents heat transfer in or out is called a calorimeter, and the use of a. Calorimetry Wikipedia.
From thechemistrynotes.com
Calorimeter Definition, Types and Uses Calorimetry Wikipedia a calorimeter is a tool that measures the amount of thermal energy contained in a substance, or released in a reaction. a container that prevents heat transfer in or out is called a calorimeter, and the use of a calorimeter to make measurements (typically of heat or specific heat. a calorimeter is a device used for calorimetry,. Calorimetry Wikipedia.
From grade12uchemistry.weebly.com
Calorimetry Grade12UChemistry Calorimetry Wikipedia a calorimeter is a tool that measures the amount of thermal energy contained in a substance, or released in a reaction. a calorimeter is a device used for calorimetry, or the process of measuring the heat of chemical reactions or physical. differential scanning calorimetry (dsc) is a thermoanalytical technique in which the difference in the amount of. Calorimetry Wikipedia.
From www.gbu-presnenskij.ru
Isothermal Titration Calorimetry Wikipedia, 51 OFF Calorimetry Wikipedia calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat. a calorimeter is a tool that measures the amount of thermal energy contained in a substance, or released in a reaction. a calorimeter is a device used for calorimetry, or the process of measuring the heat of chemical. Calorimetry Wikipedia.
From studylib.net
Calorimetry Calorimetry Wikipedia calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. differential scanning calorimetry (dsc) is a thermoanalytical technique in which the difference in the amount of heat required. a container that prevents heat transfer in or out is called a calorimeter, and the use of a calorimeter to make measurements. Calorimetry Wikipedia.
From www.thoughtco.com
Calorimeter Definition in Chemistry Calorimetry Wikipedia a calorimeter is a tool that measures the amount of thermal energy contained in a substance, or released in a reaction. calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat. calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction.. Calorimetry Wikipedia.
From www.slideserve.com
PPT CALORIMETRY PowerPoint Presentation, free download ID5767247 Calorimetry Wikipedia a calorimeter is a device used for calorimetry, or the process of measuring the heat of chemical reactions or physical. a container that prevents heat transfer in or out is called a calorimeter, and the use of a calorimeter to make measurements (typically of heat or specific heat. calorimetry is the process of measuring the amount of. Calorimetry Wikipedia.
From www.youtube.com
050 Calorimetry YouTube Calorimetry Wikipedia a calorimeter is a tool that measures the amount of thermal energy contained in a substance, or released in a reaction. calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat.. Calorimetry Wikipedia.
From www.collegesearch.in
Principle of Calorimetry Definition, Formula, Principle, Types Calorimetry Wikipedia a calorimeter is a tool that measures the amount of thermal energy contained in a substance, or released in a reaction. calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat. a calorimeter is a device used for calorimetry, or the process of measuring the heat of chemical. Calorimetry Wikipedia.
From en.wikipedia.org
Isothermal titration calorimetry Wikipedia Calorimetry Wikipedia calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat. calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. a container that prevents heat transfer in or out is called a calorimeter, and the use of a calorimeter to make. Calorimetry Wikipedia.
From www.vrogue.co
Differential Scanning Calorimetry Wikipedia vrogue.co Calorimetry Wikipedia a calorimeter is a tool that measures the amount of thermal energy contained in a substance, or released in a reaction. calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat. a container that prevents heat transfer in or out is called a calorimeter, and the use of. Calorimetry Wikipedia.
From courses.lumenlearning.com
Calorimetry Chemistry I Calorimetry Wikipedia calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat. a container that prevents heat transfer in or out is called a calorimeter, and the use of a calorimeter to make measurements (typically of heat or specific heat. differential scanning calorimetry (dsc) is a thermoanalytical technique in which. Calorimetry Wikipedia.
From www.vrogue.co
Differential Scanning Calorimetry Wikipedia vrogue.co Calorimetry Wikipedia calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. a calorimeter is a device used for calorimetry, or the process of measuring the heat of chemical reactions or physical. calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat. . Calorimetry Wikipedia.
From www.vedantu.com
Bomb Calorimeter Learn Important Terms and Concepts Calorimetry Wikipedia a calorimeter is a device used for calorimetry, or the process of measuring the heat of chemical reactions or physical. calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat. a container that prevents heat transfer in or out is called a calorimeter, and the use of a. Calorimetry Wikipedia.
From www.savemyexams.com
Calorimetry Experiments SL IB Chemistry Revision Notes 2025 Calorimetry Wikipedia calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. a calorimeter is a device used for calorimetry, or the process of measuring the heat of chemical reactions or physical. a calorimeter is a tool that measures the amount of thermal energy contained in a substance, or released in a. Calorimetry Wikipedia.
From www.vrogue.co
Differential Scanning Calorimetry Wikipedia vrogue.co Calorimetry Wikipedia differential scanning calorimetry (dsc) is a thermoanalytical technique in which the difference in the amount of heat required. calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat. a calorimeter. Calorimetry Wikipedia.
From saylordotorg.github.io
Calorimetry Calorimetry Wikipedia calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. a calorimeter is a device used for calorimetry, or the process of measuring the heat of chemical reactions or physical. a container that prevents heat transfer in or out is called a calorimeter, and the use of a calorimeter to. Calorimetry Wikipedia.
From www.scribd.com
Cone Calorimeter Wikipedia PDF Chemistry Heat Transfer Calorimetry Wikipedia a calorimeter is a device used for calorimetry, or the process of measuring the heat of chemical reactions or physical. a calorimeter is a tool that measures the amount of thermal energy contained in a substance, or released in a reaction. a container that prevents heat transfer in or out is called a calorimeter, and the use. Calorimetry Wikipedia.