Calorimetry Equation For Mass . When two objects initially at different temperatures are placed in contact, we can use equation \(\ref{5.5.7}\) to calculate the final. Calorimetry is used to measure amounts of heat transferred to or from a substance. It can analyze the heat exchange between up to 3 objects. Calorimetry is used to measure. Use our revision notes on calorimetry for a level chemistry to understand how to measure enthalpy changes and calculate specific heat capacity. The heat lost by the pan is equal to the heat gained by the water—that is the basic principle of calorimetry. Compare and contrast coffee cup calorimetry and bomb calorimetry. The calorimetry calculator can help you solve complex calorimetry problems. Calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two substances in a calorimeter. Calculate the molar heat of enthalpy for a reactions using coffee cup calorimetry. Additionally, it can find the. Use the equation for heat transfer \(q = mc\delta t\) to express the. One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. To do so, the heat is exchanged with a calibrated object. Q signifies the heat energy transferred.
from ar.inspiredpencil.com
Calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two substances in a calorimeter. Compare and contrast coffee cup calorimetry and bomb calorimetry. Calorimetry is used to measure. Q signifies the heat energy transferred. When two objects initially at different temperatures are placed in contact, we can use equation \(\ref{5.5.7}\) to calculate the final. Use our revision notes on calorimetry for a level chemistry to understand how to measure enthalpy changes and calculate specific heat capacity. To do so, the heat is exchanged with a calibrated object. One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. The heat lost by the pan is equal to the heat gained by the water—that is the basic principle of calorimetry. Calculate the molar heat of enthalpy for a reactions using coffee cup calorimetry.
Calorimetry Equation
Calorimetry Equation For Mass Compare and contrast coffee cup calorimetry and bomb calorimetry. Calorimetry is used to measure amounts of heat transferred to or from a substance. Q signifies the heat energy transferred. Use the equation for heat transfer \(q = mc\delta t\) to express the. It can analyze the heat exchange between up to 3 objects. The calorimetry calculator can help you solve complex calorimetry problems. M is the mass of the substance. Additionally, it can find the. One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. When two objects initially at different temperatures are placed in contact, we can use equation \(\ref{5.5.7}\) to calculate the final. Use our revision notes on calorimetry for a level chemistry to understand how to measure enthalpy changes and calculate specific heat capacity. Calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two substances in a calorimeter. Compare and contrast coffee cup calorimetry and bomb calorimetry. Calculate the molar heat of enthalpy for a reactions using coffee cup calorimetry. Calorimetry is used to measure. To do so, the heat is exchanged with a calibrated object.
From www.numerade.com
SOLVEDThe defining equation for calorimetry is Calorimetry Equation For Mass Calculate the molar heat of enthalpy for a reactions using coffee cup calorimetry. Additionally, it can find the. Calorimetry is used to measure amounts of heat transferred to or from a substance. Compare and contrast coffee cup calorimetry and bomb calorimetry. Use our revision notes on calorimetry for a level chemistry to understand how to measure enthalpy changes and calculate. Calorimetry Equation For Mass.
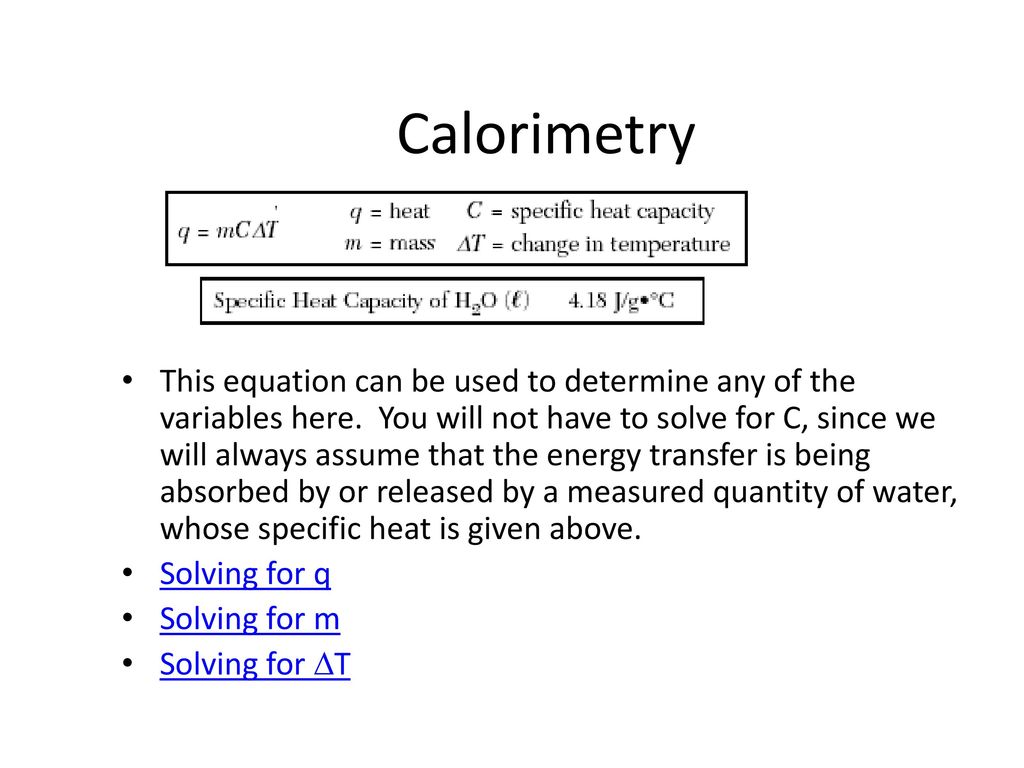
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation ID1609320 Calorimetry Equation For Mass One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. Calorimetry is used to measure. The calorimetry calculator can help you solve complex calorimetry problems. Calculate the molar heat of enthalpy for a reactions using coffee cup calorimetry. The heat lost by the pan is equal to the. Calorimetry Equation For Mass.
From www.youtube.com
How to find calorimeter constant YouTube Calorimetry Equation For Mass The calorimetry calculator can help you solve complex calorimetry problems. To do so, the heat is exchanged with a calibrated object. Use the equation for heat transfer \(q = mc\delta t\) to express the. It can analyze the heat exchange between up to 3 objects. Additionally, it can find the. Calorimetry is used to measure amounts of heat transferred to. Calorimetry Equation For Mass.
From www.tessshebaylo.com
Equation For Calorimetry Tessshebaylo Calorimetry Equation For Mass Calculate the molar heat of enthalpy for a reactions using coffee cup calorimetry. Use the equation for heat transfer \(q = mc\delta t\) to express the. One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. The heat lost by the pan is equal to the heat gained. Calorimetry Equation For Mass.
From www.youtube.com
Using Calorimetry to Calculate Enthalpies of Reaction Chemistry Calorimetry Equation For Mass Calculate the molar heat of enthalpy for a reactions using coffee cup calorimetry. It can analyze the heat exchange between up to 3 objects. Additionally, it can find the. One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. Use our revision notes on calorimetry for a level. Calorimetry Equation For Mass.
From www.youtube.com
How To Solve Basic Calorimetry Problems in Chemistry YouTube Calorimetry Equation For Mass The heat lost by the pan is equal to the heat gained by the water—that is the basic principle of calorimetry. M is the mass of the substance. When two objects initially at different temperatures are placed in contact, we can use equation \(\ref{5.5.7}\) to calculate the final. Q signifies the heat energy transferred. Additionally, it can find the. Calorimetry. Calorimetry Equation For Mass.
From www.slideserve.com
PPT Chapter 10 PowerPoint Presentation, free download ID1913481 Calorimetry Equation For Mass Calculate the molar heat of enthalpy for a reactions using coffee cup calorimetry. Calorimetry is used to measure amounts of heat transferred to or from a substance. M is the mass of the substance. Use our revision notes on calorimetry for a level chemistry to understand how to measure enthalpy changes and calculate specific heat capacity. The calorimetry calculator can. Calorimetry Equation For Mass.
From www.slideserve.com
PPT Chapter 17 PowerPoint Presentation, free download ID1792465 Calorimetry Equation For Mass The calorimetry calculator can help you solve complex calorimetry problems. Compare and contrast coffee cup calorimetry and bomb calorimetry. Calorimetry is used to measure amounts of heat transferred to or from a substance. Use our revision notes on calorimetry for a level chemistry to understand how to measure enthalpy changes and calculate specific heat capacity. Additionally, it can find the.. Calorimetry Equation For Mass.
From www.youtube.com
Specific Heat Capacity Short Example Solving for Mass YouTube Calorimetry Equation For Mass It can analyze the heat exchange between up to 3 objects. Additionally, it can find the. Calorimetry is used to measure. When two objects initially at different temperatures are placed in contact, we can use equation \(\ref{5.5.7}\) to calculate the final. Calculate the molar heat of enthalpy for a reactions using coffee cup calorimetry. The heat lost by the pan. Calorimetry Equation For Mass.
From printablezonemarrow.z13.web.core.windows.net
How To Calculate Heat Of Reaction Calorimetry Calorimetry Equation For Mass Calculate the molar heat of enthalpy for a reactions using coffee cup calorimetry. Calorimetry is used to measure amounts of heat transferred to or from a substance. M is the mass of the substance. Q signifies the heat energy transferred. Calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two substances in a calorimeter. To do. Calorimetry Equation For Mass.
From www.slideserve.com
PPT Honors Physics PowerPoint Presentation, free download ID3195016 Calorimetry Equation For Mass Use the equation for heat transfer \(q = mc\delta t\) to express the. Use our revision notes on calorimetry for a level chemistry to understand how to measure enthalpy changes and calculate specific heat capacity. Q signifies the heat energy transferred. Additionally, it can find the. The calorimetry calculator can help you solve complex calorimetry problems. Compare and contrast coffee. Calorimetry Equation For Mass.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation, free download ID6133898 Calorimetry Equation For Mass Compare and contrast coffee cup calorimetry and bomb calorimetry. It can analyze the heat exchange between up to 3 objects. Calorimetry is used to measure amounts of heat transferred to or from a substance. Calculate the molar heat of enthalpy for a reactions using coffee cup calorimetry. Use the equation for heat transfer \(q = mc\delta t\) to express the.. Calorimetry Equation For Mass.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation, free download ID6912350 Calorimetry Equation For Mass Calculate the molar heat of enthalpy for a reactions using coffee cup calorimetry. The calorimetry calculator can help you solve complex calorimetry problems. Compare and contrast coffee cup calorimetry and bomb calorimetry. Additionally, it can find the. Use our revision notes on calorimetry for a level chemistry to understand how to measure enthalpy changes and calculate specific heat capacity. To. Calorimetry Equation For Mass.
From www.youtube.com
Calorimetry calculation YouTube Calorimetry Equation For Mass Calorimetry is used to measure amounts of heat transferred to or from a substance. When two objects initially at different temperatures are placed in contact, we can use equation \(\ref{5.5.7}\) to calculate the final. It can analyze the heat exchange between up to 3 objects. Calorimetry is used to measure. The calorimetry calculator can help you solve complex calorimetry problems.. Calorimetry Equation For Mass.
From www.tessshebaylo.com
Equation For Calorimetry Tessshebaylo Calorimetry Equation For Mass Use our revision notes on calorimetry for a level chemistry to understand how to measure enthalpy changes and calculate specific heat capacity. One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. When two objects initially at different temperatures are placed in contact, we can use equation \(\ref{5.5.7}\). Calorimetry Equation For Mass.
From www.youtube.com
CHEMISTRY 101 Calculating Change in Internal Energy Using Constant Calorimetry Equation For Mass Q signifies the heat energy transferred. The heat lost by the pan is equal to the heat gained by the water—that is the basic principle of calorimetry. Calorimetry is used to measure. Use our revision notes on calorimetry for a level chemistry to understand how to measure enthalpy changes and calculate specific heat capacity. M is the mass of the. Calorimetry Equation For Mass.
From www.youtube.com
Physics 9.09g Calorimetry Example 2 YouTube Calorimetry Equation For Mass It can analyze the heat exchange between up to 3 objects. One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. Compare and contrast coffee cup calorimetry and bomb calorimetry. M is the mass of the substance. Additionally, it can find the. Q signifies the heat energy transferred.. Calorimetry Equation For Mass.
From www.chemistrystudent.com
Calorimetry (ALevel) ChemistryStudent Calorimetry Equation For Mass Use our revision notes on calorimetry for a level chemistry to understand how to measure enthalpy changes and calculate specific heat capacity. One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. It can analyze the heat exchange between up to 3 objects. Calorimetry is used to measure.. Calorimetry Equation For Mass.
From slideplayer.com
Calorimetry. ppt download Calorimetry Equation For Mass Use our revision notes on calorimetry for a level chemistry to understand how to measure enthalpy changes and calculate specific heat capacity. Calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two substances in a calorimeter. Additionally, it can find the. The heat lost by the pan is equal to the heat gained by the water—that. Calorimetry Equation For Mass.
From www.showme.com
4 steps for solving calorimetry problems Science, Chemistry ShowMe Calorimetry Equation For Mass Compare and contrast coffee cup calorimetry and bomb calorimetry. Q signifies the heat energy transferred. The calorimetry calculator can help you solve complex calorimetry problems. The heat lost by the pan is equal to the heat gained by the water—that is the basic principle of calorimetry. Calculate the molar heat of enthalpy for a reactions using coffee cup calorimetry. To. Calorimetry Equation For Mass.
From www.youtube.com
Calorimetry Part 2 calculations YouTube Calorimetry Equation For Mass The heat lost by the pan is equal to the heat gained by the water—that is the basic principle of calorimetry. M is the mass of the substance. Q signifies the heat energy transferred. Calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two substances in a calorimeter. Calorimetry is used to measure. To do so,. Calorimetry Equation For Mass.
From www.tessshebaylo.com
Equation For Calorimetry Tessshebaylo Calorimetry Equation For Mass Compare and contrast coffee cup calorimetry and bomb calorimetry. The heat lost by the pan is equal to the heat gained by the water—that is the basic principle of calorimetry. Calorimetry is used to measure amounts of heat transferred to or from a substance. Q signifies the heat energy transferred. Calculate heat, temperature change, and specific heat after thermal equilibrium. Calorimetry Equation For Mass.
From www.gbu-presnenskij.ru
Chapter 25 Essential Calorimetry Formulae James Kennedy, 47 OFF Calorimetry Equation For Mass Q signifies the heat energy transferred. Calorimetry is used to measure amounts of heat transferred to or from a substance. Use our revision notes on calorimetry for a level chemistry to understand how to measure enthalpy changes and calculate specific heat capacity. It can analyze the heat exchange between up to 3 objects. Calorimetry is used to measure. M is. Calorimetry Equation For Mass.
From www.chemistrystudent.com
Calorimetry (ALevel) ChemistryStudent Calorimetry Equation For Mass Compare and contrast coffee cup calorimetry and bomb calorimetry. It can analyze the heat exchange between up to 3 objects. Calculate the molar heat of enthalpy for a reactions using coffee cup calorimetry. Use our revision notes on calorimetry for a level chemistry to understand how to measure enthalpy changes and calculate specific heat capacity. The calorimetry calculator can help. Calorimetry Equation For Mass.
From www.youtube.com
AP Chemistry Thermochemical Equations and Calorimetry YouTube Calorimetry Equation For Mass Calorimetry is used to measure. One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. The calorimetry calculator can help you solve complex calorimetry problems. It can analyze the heat exchange between up to 3 objects. Compare and contrast coffee cup calorimetry and bomb calorimetry. Calculate heat, temperature. Calorimetry Equation For Mass.
From www.youtube.com
AP Chemistry Thermochemistry I Part 4 Constant Pressure Calorimetry Calorimetry Equation For Mass When two objects initially at different temperatures are placed in contact, we can use equation \(\ref{5.5.7}\) to calculate the final. Calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two substances in a calorimeter. The heat lost by the pan is equal to the heat gained by the water—that is the basic principle of calorimetry. It. Calorimetry Equation For Mass.
From www.studypool.com
SOLUTION Calorimetry formula sheet Studypool Calorimetry Equation For Mass When two objects initially at different temperatures are placed in contact, we can use equation \(\ref{5.5.7}\) to calculate the final. Compare and contrast coffee cup calorimetry and bomb calorimetry. Additionally, it can find the. Calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two substances in a calorimeter. The heat lost by the pan is equal. Calorimetry Equation For Mass.
From www.youtube.com
Physics 9.09b Calorimetry Example 1 YouTube Calorimetry Equation For Mass Calculate the molar heat of enthalpy for a reactions using coffee cup calorimetry. Calorimetry is used to measure. Calorimetry is used to measure amounts of heat transferred to or from a substance. One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. When two objects initially at different. Calorimetry Equation For Mass.
From lessonlibrarydetrudes.z21.web.core.windows.net
How To Solve Calorimetry Problems Chemistry Calorimetry Equation For Mass Calorimetry is used to measure amounts of heat transferred to or from a substance. One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. To do so, the heat is exchanged with a calibrated object. It can analyze the heat exchange between up to 3 objects. The calorimetry. Calorimetry Equation For Mass.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation, free download ID3206916 Calorimetry Equation For Mass It can analyze the heat exchange between up to 3 objects. Calorimetry is used to measure. M is the mass of the substance. Q signifies the heat energy transferred. Additionally, it can find the. The calorimetry calculator can help you solve complex calorimetry problems. Compare and contrast coffee cup calorimetry and bomb calorimetry. Calculate heat, temperature change, and specific heat. Calorimetry Equation For Mass.
From ar.inspiredpencil.com
Calorimetry Equation Calorimetry Equation For Mass Calculate the molar heat of enthalpy for a reactions using coffee cup calorimetry. Use our revision notes on calorimetry for a level chemistry to understand how to measure enthalpy changes and calculate specific heat capacity. Calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two substances in a calorimeter. Compare and contrast coffee cup calorimetry and. Calorimetry Equation For Mass.
From www.slideserve.com
PPT Unit 13 Thermochemistry PowerPoint Presentation, free download Calorimetry Equation For Mass M is the mass of the substance. Calorimetry is used to measure. Calorimetry is used to measure amounts of heat transferred to or from a substance. Calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two substances in a calorimeter. Use our revision notes on calorimetry for a level chemistry to understand how to measure enthalpy. Calorimetry Equation For Mass.
From slideplayer.com
Calorimetry Chapter ppt download Calorimetry Equation For Mass Calculate the molar heat of enthalpy for a reactions using coffee cup calorimetry. The calorimetry calculator can help you solve complex calorimetry problems. Use our revision notes on calorimetry for a level chemistry to understand how to measure enthalpy changes and calculate specific heat capacity. Q signifies the heat energy transferred. One technique we can use to measure the amount. Calorimetry Equation For Mass.
From fyohqoqgp.blob.core.windows.net
Calorimeter Formula In Physics at Caroline Graig blog Calorimetry Equation For Mass M is the mass of the substance. It can analyze the heat exchange between up to 3 objects. The calorimetry calculator can help you solve complex calorimetry problems. Additionally, it can find the. Q signifies the heat energy transferred. Use our revision notes on calorimetry for a level chemistry to understand how to measure enthalpy changes and calculate specific heat. Calorimetry Equation For Mass.
From worksheetmediadwaum.z14.web.core.windows.net
How To Calculate Calorimetry Problems Calorimetry Equation For Mass When two objects initially at different temperatures are placed in contact, we can use equation \(\ref{5.5.7}\) to calculate the final. Calorimetry is used to measure amounts of heat transferred to or from a substance. M is the mass of the substance. Calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two substances in a calorimeter. Additionally,. Calorimetry Equation For Mass.