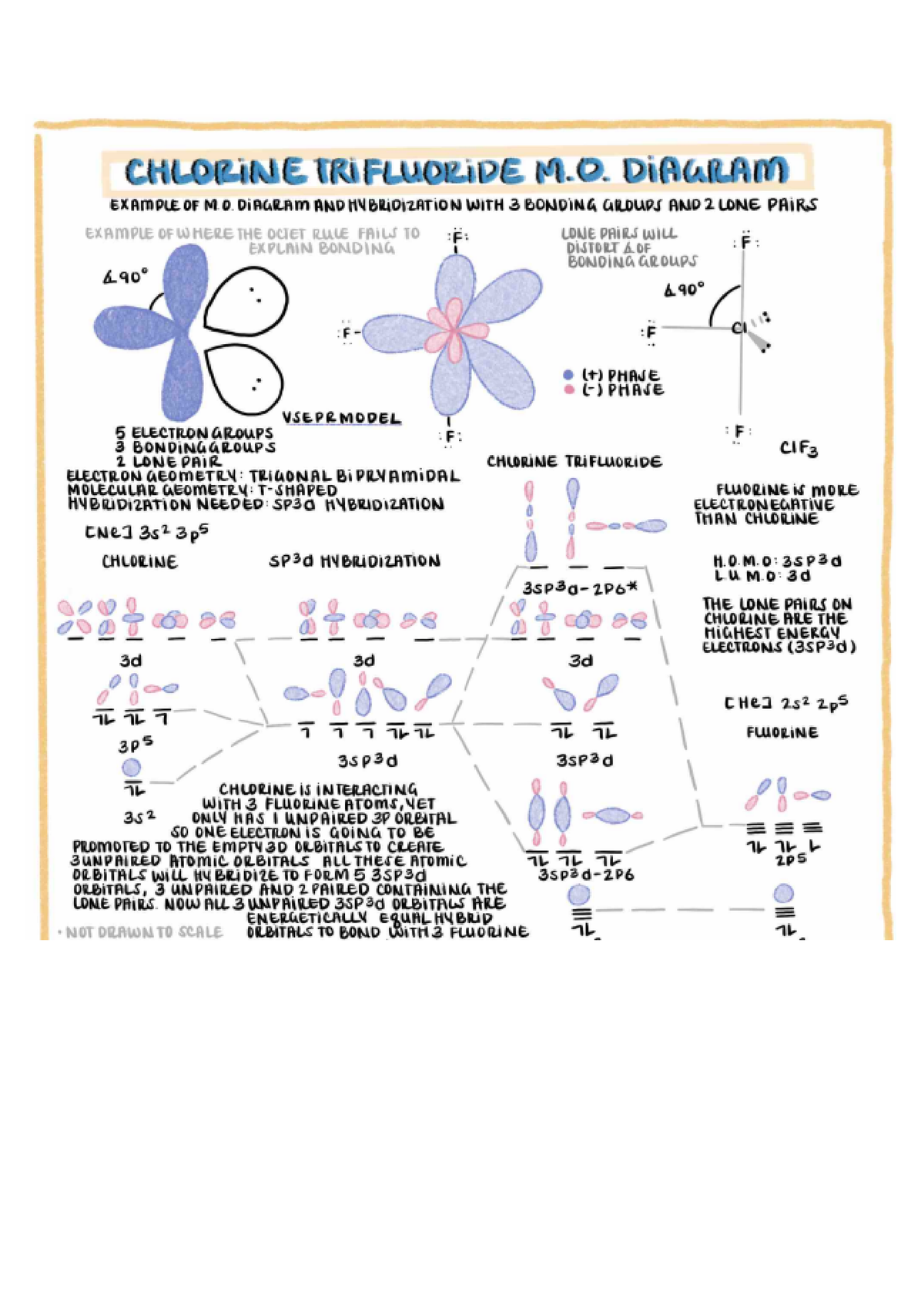
Chlorine Trifluoride Polarity . Is clf3 polar or nonpolar? This is due to the asymmetric arrangement of the fluorine atoms and lone pairs around the central chlorine atom, which results in a net dipole moment, indicating the molecule’s polarity. A chlorine atom has an electronegativity of 3.16 whereas a fluorine atom, being highly electronegative has a value of. Clf3 is a polar molecule because it has an asymmetrical shape and the presence of 2 lone pair electrons. Clf3 is a polar molecule.
from www.studocu.com
This is due to the asymmetric arrangement of the fluorine atoms and lone pairs around the central chlorine atom, which results in a net dipole moment, indicating the molecule’s polarity. A chlorine atom has an electronegativity of 3.16 whereas a fluorine atom, being highly electronegative has a value of. Clf3 is a polar molecule. Is clf3 polar or nonpolar? Clf3 is a polar molecule because it has an asymmetrical shape and the presence of 2 lone pair electrons.
Chlorine Trifluoride M.O. Diagram CHEM12A Studocu
Chlorine Trifluoride Polarity Is clf3 polar or nonpolar? A chlorine atom has an electronegativity of 3.16 whereas a fluorine atom, being highly electronegative has a value of. Clf3 is a polar molecule. Is clf3 polar or nonpolar? This is due to the asymmetric arrangement of the fluorine atoms and lone pairs around the central chlorine atom, which results in a net dipole moment, indicating the molecule’s polarity. Clf3 is a polar molecule because it has an asymmetrical shape and the presence of 2 lone pair electrons.
From www.studocu.com
Chlorine Trifluoride M.O. Diagram CHEM12A Studocu Chlorine Trifluoride Polarity Clf3 is a polar molecule. A chlorine atom has an electronegativity of 3.16 whereas a fluorine atom, being highly electronegative has a value of. This is due to the asymmetric arrangement of the fluorine atoms and lone pairs around the central chlorine atom, which results in a net dipole moment, indicating the molecule’s polarity. Clf3 is a polar molecule because. Chlorine Trifluoride Polarity.
From www.wikiwand.com
Chlorine trifluoride oxide Wikiwand Chlorine Trifluoride Polarity Clf3 is a polar molecule. This is due to the asymmetric arrangement of the fluorine atoms and lone pairs around the central chlorine atom, which results in a net dipole moment, indicating the molecule’s polarity. Clf3 is a polar molecule because it has an asymmetrical shape and the presence of 2 lone pair electrons. A chlorine atom has an electronegativity. Chlorine Trifluoride Polarity.
From www.numerade.com
SOLVED 5) Chlorine trifluoride has two sets of lone pairs of electrons Chlorine Trifluoride Polarity A chlorine atom has an electronegativity of 3.16 whereas a fluorine atom, being highly electronegative has a value of. Clf3 is a polar molecule. Clf3 is a polar molecule because it has an asymmetrical shape and the presence of 2 lone pair electrons. Is clf3 polar or nonpolar? This is due to the asymmetric arrangement of the fluorine atoms and. Chlorine Trifluoride Polarity.
From www.chemistry-reference.com
CHLORINE TRIFLUORIDE (7790912) Physical Properties • Chemical Chlorine Trifluoride Polarity Clf3 is a polar molecule. Clf3 is a polar molecule because it has an asymmetrical shape and the presence of 2 lone pair electrons. This is due to the asymmetric arrangement of the fluorine atoms and lone pairs around the central chlorine atom, which results in a net dipole moment, indicating the molecule’s polarity. A chlorine atom has an electronegativity. Chlorine Trifluoride Polarity.
From www.researchgate.net
Samples after exposure to 1 chlorine trifluoride gas at 500 °C for Chlorine Trifluoride Polarity This is due to the asymmetric arrangement of the fluorine atoms and lone pairs around the central chlorine atom, which results in a net dipole moment, indicating the molecule’s polarity. Clf3 is a polar molecule. Is clf3 polar or nonpolar? Clf3 is a polar molecule because it has an asymmetrical shape and the presence of 2 lone pair electrons. A. Chlorine Trifluoride Polarity.
From www.slideserve.com
PPT VSEPR. PowerPoint Presentation ID214953 Chlorine Trifluoride Polarity This is due to the asymmetric arrangement of the fluorine atoms and lone pairs around the central chlorine atom, which results in a net dipole moment, indicating the molecule’s polarity. Is clf3 polar or nonpolar? Clf3 is a polar molecule because it has an asymmetrical shape and the presence of 2 lone pair electrons. A chlorine atom has an electronegativity. Chlorine Trifluoride Polarity.
From favpng.com
Chlorine Trifluoride Dichlorine Monoxide Chemistry Chloride, PNG Chlorine Trifluoride Polarity Is clf3 polar or nonpolar? Clf3 is a polar molecule because it has an asymmetrical shape and the presence of 2 lone pair electrons. This is due to the asymmetric arrangement of the fluorine atoms and lone pairs around the central chlorine atom, which results in a net dipole moment, indicating the molecule’s polarity. A chlorine atom has an electronegativity. Chlorine Trifluoride Polarity.
From www.researchgate.net
Fluorine indiffusion and peeling of PPyC layer at various chlorine Chlorine Trifluoride Polarity Clf3 is a polar molecule. Clf3 is a polar molecule because it has an asymmetrical shape and the presence of 2 lone pair electrons. A chlorine atom has an electronegativity of 3.16 whereas a fluorine atom, being highly electronegative has a value of. Is clf3 polar or nonpolar? This is due to the asymmetric arrangement of the fluorine atoms and. Chlorine Trifluoride Polarity.
From www.youtube.com
Molecular Structure of Chlorine Trifluoride Using VSEPR Theory Nature Chlorine Trifluoride Polarity Clf3 is a polar molecule. Is clf3 polar or nonpolar? This is due to the asymmetric arrangement of the fluorine atoms and lone pairs around the central chlorine atom, which results in a net dipole moment, indicating the molecule’s polarity. A chlorine atom has an electronegativity of 3.16 whereas a fluorine atom, being highly electronegative has a value of. Clf3. Chlorine Trifluoride Polarity.
From www.learnchemistry12.com
ثلاثي فلوريد الكلور Chlorine trifluoride من أخطر المواد الكيميائية Chlorine Trifluoride Polarity Is clf3 polar or nonpolar? A chlorine atom has an electronegativity of 3.16 whereas a fluorine atom, being highly electronegative has a value of. Clf3 is a polar molecule because it has an asymmetrical shape and the presence of 2 lone pair electrons. Clf3 is a polar molecule. This is due to the asymmetric arrangement of the fluorine atoms and. Chlorine Trifluoride Polarity.
From brainly.in
Draw structure of chlorine trifluoride Brainly.in Chlorine Trifluoride Polarity This is due to the asymmetric arrangement of the fluorine atoms and lone pairs around the central chlorine atom, which results in a net dipole moment, indicating the molecule’s polarity. Is clf3 polar or nonpolar? Clf3 is a polar molecule. A chlorine atom has an electronegativity of 3.16 whereas a fluorine atom, being highly electronegative has a value of. Clf3. Chlorine Trifluoride Polarity.
From readingandwritingprojectcom.web.fc2.com
what is the electronic geometry of clf3 Chlorine Trifluoride Polarity Is clf3 polar or nonpolar? This is due to the asymmetric arrangement of the fluorine atoms and lone pairs around the central chlorine atom, which results in a net dipole moment, indicating the molecule’s polarity. A chlorine atom has an electronegativity of 3.16 whereas a fluorine atom, being highly electronegative has a value of. Clf3 is a polar molecule. Clf3. Chlorine Trifluoride Polarity.
From www.slideserve.com
PPT VSEPR and Chemical Polarity PowerPoint Presentation, free Chlorine Trifluoride Polarity Is clf3 polar or nonpolar? Clf3 is a polar molecule. A chlorine atom has an electronegativity of 3.16 whereas a fluorine atom, being highly electronegative has a value of. This is due to the asymmetric arrangement of the fluorine atoms and lone pairs around the central chlorine atom, which results in a net dipole moment, indicating the molecule’s polarity. Clf3. Chlorine Trifluoride Polarity.
From www.bigstockphoto.com
Chlorine Trifluoride Image & Photo (Free Trial) Bigstock Chlorine Trifluoride Polarity Clf3 is a polar molecule because it has an asymmetrical shape and the presence of 2 lone pair electrons. Is clf3 polar or nonpolar? This is due to the asymmetric arrangement of the fluorine atoms and lone pairs around the central chlorine atom, which results in a net dipole moment, indicating the molecule’s polarity. A chlorine atom has an electronegativity. Chlorine Trifluoride Polarity.
From www.bigstockphoto.com
Chlorine Trifluoride Image & Photo (Free Trial) Bigstock Chlorine Trifluoride Polarity A chlorine atom has an electronegativity of 3.16 whereas a fluorine atom, being highly electronegative has a value of. Clf3 is a polar molecule. This is due to the asymmetric arrangement of the fluorine atoms and lone pairs around the central chlorine atom, which results in a net dipole moment, indicating the molecule’s polarity. Clf3 is a polar molecule because. Chlorine Trifluoride Polarity.
From www.alamy.com
Chlorine trifluoride hires stock photography and images Alamy Chlorine Trifluoride Polarity Is clf3 polar or nonpolar? This is due to the asymmetric arrangement of the fluorine atoms and lone pairs around the central chlorine atom, which results in a net dipole moment, indicating the molecule’s polarity. Clf3 is a polar molecule because it has an asymmetrical shape and the presence of 2 lone pair electrons. A chlorine atom has an electronegativity. Chlorine Trifluoride Polarity.
From www.alamy.com
Chlorine trifluoride is an interhalogen compound with the formula ClF3 Chlorine Trifluoride Polarity This is due to the asymmetric arrangement of the fluorine atoms and lone pairs around the central chlorine atom, which results in a net dipole moment, indicating the molecule’s polarity. Clf3 is a polar molecule because it has an asymmetrical shape and the presence of 2 lone pair electrons. Is clf3 polar or nonpolar? A chlorine atom has an electronegativity. Chlorine Trifluoride Polarity.
From www.doubtnut.com
Draw structures of (a) Chlorine trifluoride (b) Chlorine pentafluori Chlorine Trifluoride Polarity Clf3 is a polar molecule. This is due to the asymmetric arrangement of the fluorine atoms and lone pairs around the central chlorine atom, which results in a net dipole moment, indicating the molecule’s polarity. Clf3 is a polar molecule because it has an asymmetrical shape and the presence of 2 lone pair electrons. A chlorine atom has an electronegativity. Chlorine Trifluoride Polarity.
From www.chegg.com
Solved 5. Molecule CIF, (chlorine trifluoride is an Chlorine Trifluoride Polarity Clf3 is a polar molecule. This is due to the asymmetric arrangement of the fluorine atoms and lone pairs around the central chlorine atom, which results in a net dipole moment, indicating the molecule’s polarity. Is clf3 polar or nonpolar? A chlorine atom has an electronegativity of 3.16 whereas a fluorine atom, being highly electronegative has a value of. Clf3. Chlorine Trifluoride Polarity.
From www.alamy.com
Chlorine trifluoride is an interhalogen compound with the formula ClF3 Chlorine Trifluoride Polarity A chlorine atom has an electronegativity of 3.16 whereas a fluorine atom, being highly electronegative has a value of. This is due to the asymmetric arrangement of the fluorine atoms and lone pairs around the central chlorine atom, which results in a net dipole moment, indicating the molecule’s polarity. Is clf3 polar or nonpolar? Clf3 is a polar molecule. Clf3. Chlorine Trifluoride Polarity.
From www.alamy.com
Chlorine trifluoride is an interhalogen compound with the formula ClF3 Chlorine Trifluoride Polarity This is due to the asymmetric arrangement of the fluorine atoms and lone pairs around the central chlorine atom, which results in a net dipole moment, indicating the molecule’s polarity. Clf3 is a polar molecule. Is clf3 polar or nonpolar? Clf3 is a polar molecule because it has an asymmetrical shape and the presence of 2 lone pair electrons. A. Chlorine Trifluoride Polarity.
From de-academic.com
Chlor(III)fluorid Chlorine Trifluoride Polarity Clf3 is a polar molecule. Clf3 is a polar molecule because it has an asymmetrical shape and the presence of 2 lone pair electrons. Is clf3 polar or nonpolar? This is due to the asymmetric arrangement of the fluorine atoms and lone pairs around the central chlorine atom, which results in a net dipole moment, indicating the molecule’s polarity. A. Chlorine Trifluoride Polarity.
From chemicalportal.ru
Фторид хлора (III) — свойства, получение и применение Chlorine Trifluoride Polarity Is clf3 polar or nonpolar? Clf3 is a polar molecule. Clf3 is a polar molecule because it has an asymmetrical shape and the presence of 2 lone pair electrons. A chlorine atom has an electronegativity of 3.16 whereas a fluorine atom, being highly electronegative has a value of. This is due to the asymmetric arrangement of the fluorine atoms and. Chlorine Trifluoride Polarity.
From www.researchgate.net
(PDF) Lowest Concentration of Chlorine Trifluoride Gas for Cleaning Chlorine Trifluoride Polarity This is due to the asymmetric arrangement of the fluorine atoms and lone pairs around the central chlorine atom, which results in a net dipole moment, indicating the molecule’s polarity. Is clf3 polar or nonpolar? Clf3 is a polar molecule because it has an asymmetrical shape and the presence of 2 lone pair electrons. Clf3 is a polar molecule. A. Chlorine Trifluoride Polarity.
From www.chegg.com
Solved Draw Lewis structures for each molecule. chlorine Chlorine Trifluoride Polarity A chlorine atom has an electronegativity of 3.16 whereas a fluorine atom, being highly electronegative has a value of. Clf3 is a polar molecule because it has an asymmetrical shape and the presence of 2 lone pair electrons. Is clf3 polar or nonpolar? This is due to the asymmetric arrangement of the fluorine atoms and lone pairs around the central. Chlorine Trifluoride Polarity.
From www.researchgate.net
Sample appearance along with exposure to chlorine trifluoride gas and Chlorine Trifluoride Polarity This is due to the asymmetric arrangement of the fluorine atoms and lone pairs around the central chlorine atom, which results in a net dipole moment, indicating the molecule’s polarity. Clf3 is a polar molecule because it has an asymmetrical shape and the presence of 2 lone pair electrons. A chlorine atom has an electronegativity of 3.16 whereas a fluorine. Chlorine Trifluoride Polarity.
From www.bigstockphoto.com
Chlorine Trifluoride Image & Photo (Free Trial) Bigstock Chlorine Trifluoride Polarity Clf3 is a polar molecule. Is clf3 polar or nonpolar? This is due to the asymmetric arrangement of the fluorine atoms and lone pairs around the central chlorine atom, which results in a net dipole moment, indicating the molecule’s polarity. Clf3 is a polar molecule because it has an asymmetrical shape and the presence of 2 lone pair electrons. A. Chlorine Trifluoride Polarity.
From www.alamy.com
Chlorine trifluoride is an interhalogen compound with the formula ClF3 Chlorine Trifluoride Polarity Clf3 is a polar molecule because it has an asymmetrical shape and the presence of 2 lone pair electrons. Clf3 is a polar molecule. This is due to the asymmetric arrangement of the fluorine atoms and lone pairs around the central chlorine atom, which results in a net dipole moment, indicating the molecule’s polarity. Is clf3 polar or nonpolar? A. Chlorine Trifluoride Polarity.
From www.youtube.com
ClF3 (Chlorine trifluoride) Molecular Geometry, Bond Angles,& Electron Chlorine Trifluoride Polarity This is due to the asymmetric arrangement of the fluorine atoms and lone pairs around the central chlorine atom, which results in a net dipole moment, indicating the molecule’s polarity. Is clf3 polar or nonpolar? A chlorine atom has an electronegativity of 3.16 whereas a fluorine atom, being highly electronegative has a value of. Clf3 is a polar molecule because. Chlorine Trifluoride Polarity.
From www.shutterstock.com
Chlorine Trifluoride Molecular Structure Formula Periodic Stock Vector Chlorine Trifluoride Polarity This is due to the asymmetric arrangement of the fluorine atoms and lone pairs around the central chlorine atom, which results in a net dipole moment, indicating the molecule’s polarity. Clf3 is a polar molecule. Clf3 is a polar molecule because it has an asymmetrical shape and the presence of 2 lone pair electrons. A chlorine atom has an electronegativity. Chlorine Trifluoride Polarity.
From bilag.xxl.no
Draw The Lewis Structure For The Chlorine Trifluoride Molecule Chlorine Trifluoride Polarity Clf3 is a polar molecule because it has an asymmetrical shape and the presence of 2 lone pair electrons. Is clf3 polar or nonpolar? Clf3 is a polar molecule. A chlorine atom has an electronegativity of 3.16 whereas a fluorine atom, being highly electronegative has a value of. This is due to the asymmetric arrangement of the fluorine atoms and. Chlorine Trifluoride Polarity.
From www.youtube.com
Is ClF3 Polar or Nonpolar (Chlorine trifluoride) YouTube Chlorine Trifluoride Polarity This is due to the asymmetric arrangement of the fluorine atoms and lone pairs around the central chlorine atom, which results in a net dipole moment, indicating the molecule’s polarity. Is clf3 polar or nonpolar? Clf3 is a polar molecule. Clf3 is a polar molecule because it has an asymmetrical shape and the presence of 2 lone pair electrons. A. Chlorine Trifluoride Polarity.
From www.studocu.com
Chemistry 101 Chapter 7 Part 3 Draw the Lewis structure of ClF₃ Chlorine Trifluoride Polarity Is clf3 polar or nonpolar? Clf3 is a polar molecule. This is due to the asymmetric arrangement of the fluorine atoms and lone pairs around the central chlorine atom, which results in a net dipole moment, indicating the molecule’s polarity. Clf3 is a polar molecule because it has an asymmetrical shape and the presence of 2 lone pair electrons. A. Chlorine Trifluoride Polarity.
From www.youtube.com
ClF3 Molecular Geometry, Bond Angles & Electron Geometry (Chlorine Chlorine Trifluoride Polarity This is due to the asymmetric arrangement of the fluorine atoms and lone pairs around the central chlorine atom, which results in a net dipole moment, indicating the molecule’s polarity. Clf3 is a polar molecule. A chlorine atom has an electronegativity of 3.16 whereas a fluorine atom, being highly electronegative has a value of. Clf3 is a polar molecule because. Chlorine Trifluoride Polarity.
From www.numerade.com
SOLVED 5) Chlorine trifluoride has two sets of lone pairs of electrons Chlorine Trifluoride Polarity Clf3 is a polar molecule. Is clf3 polar or nonpolar? This is due to the asymmetric arrangement of the fluorine atoms and lone pairs around the central chlorine atom, which results in a net dipole moment, indicating the molecule’s polarity. Clf3 is a polar molecule because it has an asymmetrical shape and the presence of 2 lone pair electrons. A. Chlorine Trifluoride Polarity.