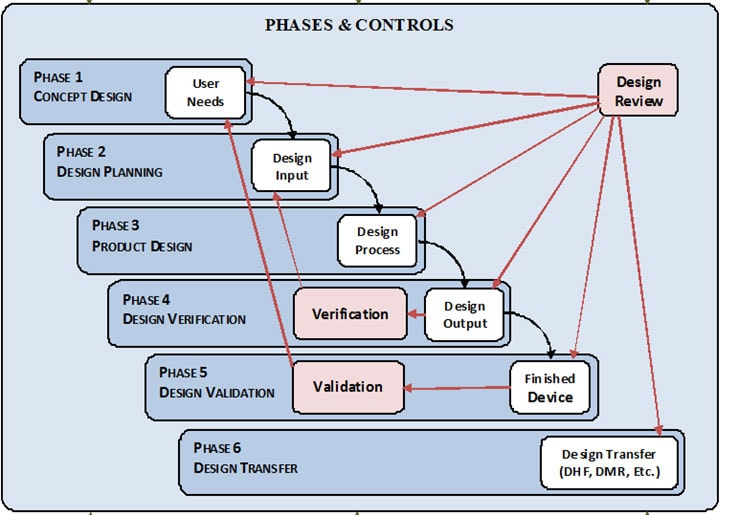
Medical Device Verification . Medical device verification guarantees that devices are designed and implemented correctly and meet the necessary standards and regulations set by the fda. Validation and verification are inextricable from product development and process design for the manufacturing of medical devices. But what’s the difference between the two, and why do we need them? Verification (1) “confirmation, through the provision of objective evidence, that specified requirements have been fulfilled.” iso 9000. Medical device verification and validation (v&v) are crucial steps in the medical device development process. What are medical device companies requesting these days when it comes to validation and verification? To put it in simpler terms, medical device design verification is determining if you built the product right, while medical device validation determines whether the right. Explains how precise verification and validation of medical devices help with approval and audits. 7.3.6 design and development verification (continued) if the intended use requires that the medical device be connected to, or have an.
from www.padtinc.com
What are medical device companies requesting these days when it comes to validation and verification? To put it in simpler terms, medical device design verification is determining if you built the product right, while medical device validation determines whether the right. 7.3.6 design and development verification (continued) if the intended use requires that the medical device be connected to, or have an. Verification (1) “confirmation, through the provision of objective evidence, that specified requirements have been fulfilled.” iso 9000. Validation and verification are inextricable from product development and process design for the manufacturing of medical devices. Explains how precise verification and validation of medical devices help with approval and audits. Medical device verification and validation (v&v) are crucial steps in the medical device development process. Medical device verification guarantees that devices are designed and implemented correctly and meet the necessary standards and regulations set by the fda. But what’s the difference between the two, and why do we need them?
FDA Opening to Simulation Supported Verification and Validation for
Medical Device Verification What are medical device companies requesting these days when it comes to validation and verification? Validation and verification are inextricable from product development and process design for the manufacturing of medical devices. 7.3.6 design and development verification (continued) if the intended use requires that the medical device be connected to, or have an. Explains how precise verification and validation of medical devices help with approval and audits. Medical device verification and validation (v&v) are crucial steps in the medical device development process. What are medical device companies requesting these days when it comes to validation and verification? Verification (1) “confirmation, through the provision of objective evidence, that specified requirements have been fulfilled.” iso 9000. But what’s the difference between the two, and why do we need them? Medical device verification guarantees that devices are designed and implemented correctly and meet the necessary standards and regulations set by the fda. To put it in simpler terms, medical device design verification is determining if you built the product right, while medical device validation determines whether the right.
From www.presentationeze.com
Medical Device Validation Requirements Principles & Practices Medical Device Verification Validation and verification are inextricable from product development and process design for the manufacturing of medical devices. Verification (1) “confirmation, through the provision of objective evidence, that specified requirements have been fulfilled.” iso 9000. What are medical device companies requesting these days when it comes to validation and verification? 7.3.6 design and development verification (continued) if the intended use requires. Medical Device Verification.
From prntbl.concejomunicipaldechinu.gov.co
Medical Device Verification And Validation Plan Template prntbl Medical Device Verification What are medical device companies requesting these days when it comes to validation and verification? To put it in simpler terms, medical device design verification is determining if you built the product right, while medical device validation determines whether the right. Medical device verification guarantees that devices are designed and implemented correctly and meet the necessary standards and regulations set. Medical Device Verification.
From www.orielstat.com
Overview of Medical Device Process Validation IQ, OQ, and PQ Oriel Medical Device Verification What are medical device companies requesting these days when it comes to validation and verification? Medical device verification and validation (v&v) are crucial steps in the medical device development process. To put it in simpler terms, medical device design verification is determining if you built the product right, while medical device validation determines whether the right. Validation and verification are. Medical Device Verification.
From www.padtinc.com
FDA Opening to Simulation Supported Verification and Validation for Medical Device Verification Medical device verification guarantees that devices are designed and implemented correctly and meet the necessary standards and regulations set by the fda. To put it in simpler terms, medical device design verification is determining if you built the product right, while medical device validation determines whether the right. Validation and verification are inextricable from product development and process design for. Medical Device Verification.
From www.qmdocs.com
Medical Device Design Verification SOP Medical Device Verification Medical device verification guarantees that devices are designed and implemented correctly and meet the necessary standards and regulations set by the fda. Verification (1) “confirmation, through the provision of objective evidence, that specified requirements have been fulfilled.” iso 9000. Validation and verification are inextricable from product development and process design for the manufacturing of medical devices. Explains how precise verification. Medical Device Verification.
From www.aplyon.com
Design Verification and Validation Procedure Medical Device Verification To put it in simpler terms, medical device design verification is determining if you built the product right, while medical device validation determines whether the right. What are medical device companies requesting these days when it comes to validation and verification? 7.3.6 design and development verification (continued) if the intended use requires that the medical device be connected to, or. Medical Device Verification.
From medicaldeviceacademy.com
Define medical device software verification and validation (V&V Medical Device Verification Medical device verification and validation (v&v) are crucial steps in the medical device development process. But what’s the difference between the two, and why do we need them? Validation and verification are inextricable from product development and process design for the manufacturing of medical devices. 7.3.6 design and development verification (continued) if the intended use requires that the medical device. Medical Device Verification.
From www.greenlight.guru
What Device Makers Need To Know About Design Verification and Validation Medical Device Verification Verification (1) “confirmation, through the provision of objective evidence, that specified requirements have been fulfilled.” iso 9000. What are medical device companies requesting these days when it comes to validation and verification? Medical device verification and validation (v&v) are crucial steps in the medical device development process. Medical device verification guarantees that devices are designed and implemented correctly and meet. Medical Device Verification.
From old.sermitsiaq.ag
Medical Device Design Review Template Medical Device Verification Validation and verification are inextricable from product development and process design for the manufacturing of medical devices. But what’s the difference between the two, and why do we need them? Verification (1) “confirmation, through the provision of objective evidence, that specified requirements have been fulfilled.” iso 9000. Explains how precise verification and validation of medical devices help with approval and. Medical Device Verification.
From easymedicaldevice.com
Process Validation or Verification (Medical Device)? Medical Device Verification Medical device verification guarantees that devices are designed and implemented correctly and meet the necessary standards and regulations set by the fda. Verification (1) “confirmation, through the provision of objective evidence, that specified requirements have been fulfilled.” iso 9000. Explains how precise verification and validation of medical devices help with approval and audits. 7.3.6 design and development verification (continued) if. Medical Device Verification.
From www.greenlight.guru
Design Verification & Validation for Medical Devices [Guide] Medical Device Verification Verification (1) “confirmation, through the provision of objective evidence, that specified requirements have been fulfilled.” iso 9000. Medical device verification and validation (v&v) are crucial steps in the medical device development process. 7.3.6 design and development verification (continued) if the intended use requires that the medical device be connected to, or have an. What are medical device companies requesting these. Medical Device Verification.
From www.upcitemdb.com
ISBN 9781596934238 Medical Device Software Verification, Validation Medical Device Verification To put it in simpler terms, medical device design verification is determining if you built the product right, while medical device validation determines whether the right. Explains how precise verification and validation of medical devices help with approval and audits. Medical device verification guarantees that devices are designed and implemented correctly and meet the necessary standards and regulations set by. Medical Device Verification.
From www.qualitymeddev.com
IEC 623042006 Medical Device Software QualityMedDev Medical Device Verification Medical device verification guarantees that devices are designed and implemented correctly and meet the necessary standards and regulations set by the fda. 7.3.6 design and development verification (continued) if the intended use requires that the medical device be connected to, or have an. What are medical device companies requesting these days when it comes to validation and verification? Medical device. Medical Device Verification.
From www.omnica.com
Medical Device Verification and Validation Omnica Corporation Medical Device Verification But what’s the difference between the two, and why do we need them? Explains how precise verification and validation of medical devices help with approval and audits. To put it in simpler terms, medical device design verification is determining if you built the product right, while medical device validation determines whether the right. Verification (1) “confirmation, through the provision of. Medical Device Verification.
From www.qmdocs.com
Medical Device Design And Development Plan QMDocs Quality Management Medical Device Verification Validation and verification are inextricable from product development and process design for the manufacturing of medical devices. Verification (1) “confirmation, through the provision of objective evidence, that specified requirements have been fulfilled.” iso 9000. Medical device verification guarantees that devices are designed and implemented correctly and meet the necessary standards and regulations set by the fda. To put it in. Medical Device Verification.
From academycenters.com
Verification & Validation of Medical Devices Medical Device Verification What are medical device companies requesting these days when it comes to validation and verification? Medical device verification guarantees that devices are designed and implemented correctly and meet the necessary standards and regulations set by the fda. Medical device verification and validation (v&v) are crucial steps in the medical device development process. 7.3.6 design and development verification (continued) if the. Medical Device Verification.
From in.pinterest.com
Verification Vs Validation ISO 9001 Definitions Medical Device Verification Validation and verification are inextricable from product development and process design for the manufacturing of medical devices. What are medical device companies requesting these days when it comes to validation and verification? Medical device verification guarantees that devices are designed and implemented correctly and meet the necessary standards and regulations set by the fda. Explains how precise verification and validation. Medical Device Verification.
From bluefruit.co.uk
V&V Testing Signs you have a verification & validation issue Medical Device Verification Explains how precise verification and validation of medical devices help with approval and audits. Verification (1) “confirmation, through the provision of objective evidence, that specified requirements have been fulfilled.” iso 9000. 7.3.6 design and development verification (continued) if the intended use requires that the medical device be connected to, or have an. Medical device verification and validation (v&v) are crucial. Medical Device Verification.
From www.kolabtree.com
Medical Device Design The Essential, StepbyStep Guide Medical Device Verification 7.3.6 design and development verification (continued) if the intended use requires that the medical device be connected to, or have an. Medical device verification guarantees that devices are designed and implemented correctly and meet the necessary standards and regulations set by the fda. But what’s the difference between the two, and why do we need them? Medical device verification and. Medical Device Verification.
From studylib.net
Medical Device Product Verification and Validation Medical Device Verification Explains how precise verification and validation of medical devices help with approval and audits. 7.3.6 design and development verification (continued) if the intended use requires that the medical device be connected to, or have an. Medical device verification and validation (v&v) are crucial steps in the medical device development process. Medical device verification guarantees that devices are designed and implemented. Medical Device Verification.
From academy.qualitymeddev.com
Medical Device Software Verification & Validation QualityMedDev Academy Medical Device Verification Verification (1) “confirmation, through the provision of objective evidence, that specified requirements have been fulfilled.” iso 9000. 7.3.6 design and development verification (continued) if the intended use requires that the medical device be connected to, or have an. What are medical device companies requesting these days when it comes to validation and verification? But what’s the difference between the two,. Medical Device Verification.
From old.sermitsiaq.ag
Medical Device Verification And Validation Plan Template Medical Device Verification Explains how precise verification and validation of medical devices help with approval and audits. 7.3.6 design and development verification (continued) if the intended use requires that the medical device be connected to, or have an. To put it in simpler terms, medical device design verification is determining if you built the product right, while medical device validation determines whether the. Medical Device Verification.
From www.proplate.com
Medical Device Design & Development Electroplating Solutions ProPlate Medical Device Verification To put it in simpler terms, medical device design verification is determining if you built the product right, while medical device validation determines whether the right. But what’s the difference between the two, and why do we need them? Medical device verification guarantees that devices are designed and implemented correctly and meet the necessary standards and regulations set by the. Medical Device Verification.
From old.sermitsiaq.ag
Medical Device Verification And Validation Plan Template Medical Device Verification Explains how precise verification and validation of medical devices help with approval and audits. But what’s the difference between the two, and why do we need them? To put it in simpler terms, medical device design verification is determining if you built the product right, while medical device validation determines whether the right. 7.3.6 design and development verification (continued) if. Medical Device Verification.
From www.orielstat.com
Medical Device Process Validation Overview & Steps Oriel STAT A MATRIX Medical Device Verification But what’s the difference between the two, and why do we need them? What are medical device companies requesting these days when it comes to validation and verification? 7.3.6 design and development verification (continued) if the intended use requires that the medical device be connected to, or have an. Medical device verification guarantees that devices are designed and implemented correctly. Medical Device Verification.
From www.orielstat.com
Medical Device Process Validation What You Need to Know Medical Device Verification Medical device verification and validation (v&v) are crucial steps in the medical device development process. 7.3.6 design and development verification (continued) if the intended use requires that the medical device be connected to, or have an. Explains how precise verification and validation of medical devices help with approval and audits. Validation and verification are inextricable from product development and process. Medical Device Verification.
From medium.com
Medical Device Verification per IEC 62304 by Sreekanth Yalavarthi Medical Device Verification What are medical device companies requesting these days when it comes to validation and verification? 7.3.6 design and development verification (continued) if the intended use requires that the medical device be connected to, or have an. Medical device verification guarantees that devices are designed and implemented correctly and meet the necessary standards and regulations set by the fda. But what’s. Medical Device Verification.
From tsquality.ch
The Future of Validation & Verification in Medical Devices Industry Medical Device Verification But what’s the difference between the two, and why do we need them? Validation and verification are inextricable from product development and process design for the manufacturing of medical devices. Medical device verification guarantees that devices are designed and implemented correctly and meet the necessary standards and regulations set by the fda. To put it in simpler terms, medical device. Medical Device Verification.
From bobonmedicaldevicesoftware.com
Software Verification vs. Validation Bob on Medical Device Software Medical Device Verification But what’s the difference between the two, and why do we need them? Validation and verification are inextricable from product development and process design for the manufacturing of medical devices. 7.3.6 design and development verification (continued) if the intended use requires that the medical device be connected to, or have an. To put it in simpler terms, medical device design. Medical Device Verification.
From easymedicaldevice.com
Process Validation or Verification (Medical Device)? Medical Device Verification To put it in simpler terms, medical device design verification is determining if you built the product right, while medical device validation determines whether the right. Validation and verification are inextricable from product development and process design for the manufacturing of medical devices. But what’s the difference between the two, and why do we need them? Explains how precise verification. Medical Device Verification.
From starfishmedical.com
Reducing Costs in Medical Device Verification and Validation StarFish Medical Device Verification To put it in simpler terms, medical device design verification is determining if you built the product right, while medical device validation determines whether the right. Validation and verification are inextricable from product development and process design for the manufacturing of medical devices. But what’s the difference between the two, and why do we need them? Explains how precise verification. Medical Device Verification.
From www.worksure.org
Medical device design verification WorkSure Medical Device Verification What are medical device companies requesting these days when it comes to validation and verification? Medical device verification guarantees that devices are designed and implemented correctly and meet the necessary standards and regulations set by the fda. Explains how precise verification and validation of medical devices help with approval and audits. Validation and verification are inextricable from product development and. Medical Device Verification.
From www.alecalpert.com
Medical Device Design Verification Essentials Alec Alpert Medical Device Verification But what’s the difference between the two, and why do we need them? Verification (1) “confirmation, through the provision of objective evidence, that specified requirements have been fulfilled.” iso 9000. Medical device verification and validation (v&v) are crucial steps in the medical device development process. To put it in simpler terms, medical device design verification is determining if you built. Medical Device Verification.
From www.scribd.com
Medical Device Design Validation SOP Verification And Validation Medical Device Verification What are medical device companies requesting these days when it comes to validation and verification? But what’s the difference between the two, and why do we need them? Explains how precise verification and validation of medical devices help with approval and audits. To put it in simpler terms, medical device design verification is determining if you built the product right,. Medical Device Verification.
From formiventos.com
Personalized Medical Devices Production Verification and Validation Medical Device Verification But what’s the difference between the two, and why do we need them? 7.3.6 design and development verification (continued) if the intended use requires that the medical device be connected to, or have an. What are medical device companies requesting these days when it comes to validation and verification? Explains how precise verification and validation of medical devices help with. Medical Device Verification.