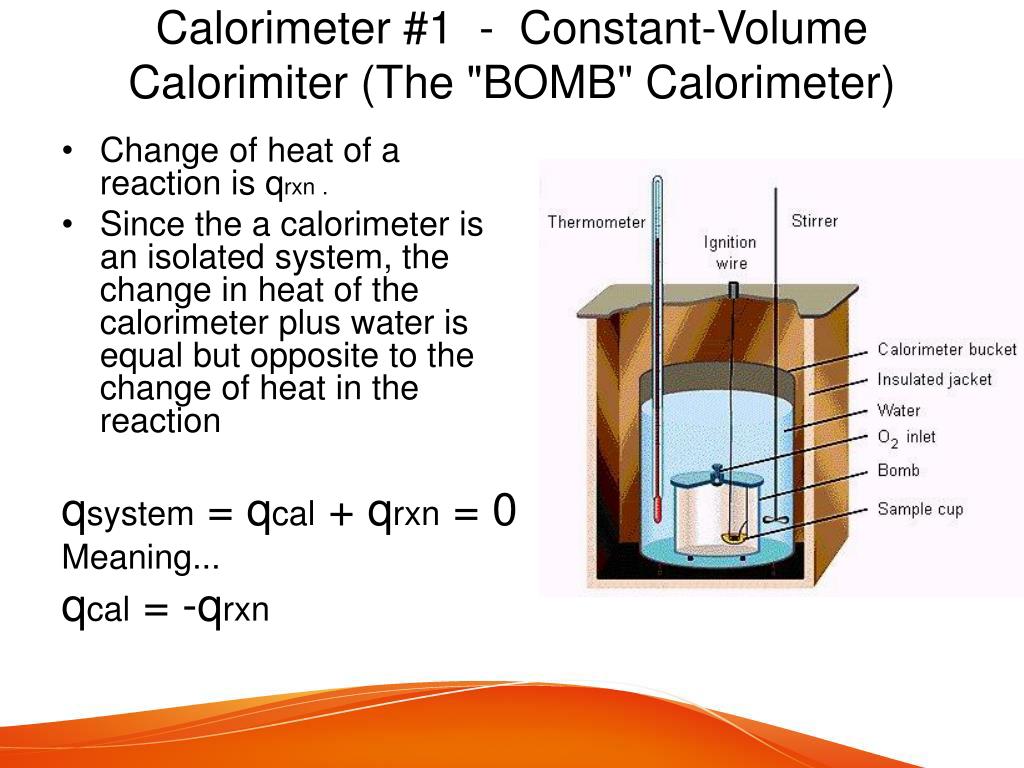
Bomb Calorimeter Constant Volume . Four essential parts are required in any bomb calorimeter: Four critical parts are needed in every bomb calorimeter. A bomb or vessel in which the combustible charges can be burned. Constant volume calorimetry, also know as bomb calorimetry, is used to measure the heat of a reaction while holding volume constant and resisting large. A bucket or container for holding the bomb in a. In chemistry, the changes of heat of a reaction can be measured at fixed pressure or volume. A bomb calorimeter operates at constant volume and is particularly useful for measuring energies of combustion. Constant volume calorimetry, also know as bomb calorimetry, is used to measure the heat of a reaction while holding volume constant and.
from www.slideserve.com
A bucket or container for holding the bomb in a. A bomb or vessel in which the combustible charges can be burned. Four essential parts are required in any bomb calorimeter: In chemistry, the changes of heat of a reaction can be measured at fixed pressure or volume. Constant volume calorimetry, also know as bomb calorimetry, is used to measure the heat of a reaction while holding volume constant and. A bomb calorimeter operates at constant volume and is particularly useful for measuring energies of combustion. Four critical parts are needed in every bomb calorimeter. Constant volume calorimetry, also know as bomb calorimetry, is used to measure the heat of a reaction while holding volume constant and resisting large.
PPT Thermochemistry PowerPoint Presentation, free download ID5194704
Bomb Calorimeter Constant Volume A bomb or vessel in which the combustible charges can be burned. Constant volume calorimetry, also know as bomb calorimetry, is used to measure the heat of a reaction while holding volume constant and. A bucket or container for holding the bomb in a. Constant volume calorimetry, also know as bomb calorimetry, is used to measure the heat of a reaction while holding volume constant and resisting large. In chemistry, the changes of heat of a reaction can be measured at fixed pressure or volume. A bomb or vessel in which the combustible charges can be burned. Four essential parts are required in any bomb calorimeter: Four critical parts are needed in every bomb calorimeter. A bomb calorimeter operates at constant volume and is particularly useful for measuring energies of combustion.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation, free download ID1875569 Bomb Calorimeter Constant Volume Constant volume calorimetry, also know as bomb calorimetry, is used to measure the heat of a reaction while holding volume constant and resisting large. Four essential parts are required in any bomb calorimeter: A bucket or container for holding the bomb in a. In chemistry, the changes of heat of a reaction can be measured at fixed pressure or volume.. Bomb Calorimeter Constant Volume.
From www.onenewspage.com
Calorimetry, Constant Volume, Bomb Calorimeter, One News Page VIDEO Bomb Calorimeter Constant Volume Four essential parts are required in any bomb calorimeter: A bucket or container for holding the bomb in a. A bomb or vessel in which the combustible charges can be burned. Four critical parts are needed in every bomb calorimeter. A bomb calorimeter operates at constant volume and is particularly useful for measuring energies of combustion. Constant volume calorimetry, also. Bomb Calorimeter Constant Volume.
From www.slideserve.com
PPT Unit 13 Thermochemistry PowerPoint Presentation, free download Bomb Calorimeter Constant Volume Constant volume calorimetry, also know as bomb calorimetry, is used to measure the heat of a reaction while holding volume constant and. In chemistry, the changes of heat of a reaction can be measured at fixed pressure or volume. A bomb calorimeter operates at constant volume and is particularly useful for measuring energies of combustion. Four essential parts are required. Bomb Calorimeter Constant Volume.
From www.chegg.com
Solved A bomb calorimeter, or a constant volume calorimeter, Bomb Calorimeter Constant Volume Constant volume calorimetry, also know as bomb calorimetry, is used to measure the heat of a reaction while holding volume constant and. Constant volume calorimetry, also know as bomb calorimetry, is used to measure the heat of a reaction while holding volume constant and resisting large. A bomb calorimeter operates at constant volume and is particularly useful for measuring energies. Bomb Calorimeter Constant Volume.
From www.chegg.com
Solved Thermometer A bomb calorimeter, or constant volume Bomb Calorimeter Constant Volume Constant volume calorimetry, also know as bomb calorimetry, is used to measure the heat of a reaction while holding volume constant and resisting large. Four critical parts are needed in every bomb calorimeter. In chemistry, the changes of heat of a reaction can be measured at fixed pressure or volume. Constant volume calorimetry, also know as bomb calorimetry, is used. Bomb Calorimeter Constant Volume.
From www.slideserve.com
PPT Thermochemistry PowerPoint Presentation, free download ID2692866 Bomb Calorimeter Constant Volume Four essential parts are required in any bomb calorimeter: A bomb calorimeter operates at constant volume and is particularly useful for measuring energies of combustion. Constant volume calorimetry, also know as bomb calorimetry, is used to measure the heat of a reaction while holding volume constant and. A bomb or vessel in which the combustible charges can be burned. A. Bomb Calorimeter Constant Volume.
From www.expii.com
Bomb Calorimeter — Structure & Function Expii Bomb Calorimeter Constant Volume Four critical parts are needed in every bomb calorimeter. In chemistry, the changes of heat of a reaction can be measured at fixed pressure or volume. Constant volume calorimetry, also know as bomb calorimetry, is used to measure the heat of a reaction while holding volume constant and resisting large. Constant volume calorimetry, also know as bomb calorimetry, is used. Bomb Calorimeter Constant Volume.
From chem.libretexts.org
6.5 Constant Volume Calorimetry Measuring ΔU for Chemical Reactions Bomb Calorimeter Constant Volume Constant volume calorimetry, also know as bomb calorimetry, is used to measure the heat of a reaction while holding volume constant and resisting large. A bucket or container for holding the bomb in a. A bomb calorimeter operates at constant volume and is particularly useful for measuring energies of combustion. In chemistry, the changes of heat of a reaction can. Bomb Calorimeter Constant Volume.
From www.youtube.com
Measuring Energy at Constant Volume Using a Bomb Calorimeter YouTube Bomb Calorimeter Constant Volume In chemistry, the changes of heat of a reaction can be measured at fixed pressure or volume. A bomb calorimeter operates at constant volume and is particularly useful for measuring energies of combustion. A bomb or vessel in which the combustible charges can be burned. Four essential parts are required in any bomb calorimeter: Four critical parts are needed in. Bomb Calorimeter Constant Volume.
From slideplayer.com
Thermodynamics Honors Unit ppt download Bomb Calorimeter Constant Volume Constant volume calorimetry, also know as bomb calorimetry, is used to measure the heat of a reaction while holding volume constant and resisting large. A bomb calorimeter operates at constant volume and is particularly useful for measuring energies of combustion. Four essential parts are required in any bomb calorimeter: Four critical parts are needed in every bomb calorimeter. A bucket. Bomb Calorimeter Constant Volume.
From www.slideserve.com
PPT Unit 13 Thermochemistry PowerPoint Presentation ID6591088 Bomb Calorimeter Constant Volume A bucket or container for holding the bomb in a. Four critical parts are needed in every bomb calorimeter. A bomb calorimeter operates at constant volume and is particularly useful for measuring energies of combustion. Constant volume calorimetry, also know as bomb calorimetry, is used to measure the heat of a reaction while holding volume constant and. Constant volume calorimetry,. Bomb Calorimeter Constant Volume.
From www.vedantu.com
Bomb Calorimeter Learn Important Terms and Concepts Bomb Calorimeter Constant Volume A bomb calorimeter operates at constant volume and is particularly useful for measuring energies of combustion. In chemistry, the changes of heat of a reaction can be measured at fixed pressure or volume. Constant volume calorimetry, also know as bomb calorimetry, is used to measure the heat of a reaction while holding volume constant and. Four critical parts are needed. Bomb Calorimeter Constant Volume.
From www.slideserve.com
PPT Energy, Enthalpy, and Thermochemistry PowerPoint Presentation Bomb Calorimeter Constant Volume A bomb calorimeter operates at constant volume and is particularly useful for measuring energies of combustion. A bomb or vessel in which the combustible charges can be burned. Four critical parts are needed in every bomb calorimeter. Constant volume calorimetry, also know as bomb calorimetry, is used to measure the heat of a reaction while holding volume constant and resisting. Bomb Calorimeter Constant Volume.
From www.slideserve.com
PPT Bomb Calorimetry PowerPoint Presentation ID3206969 Bomb Calorimeter Constant Volume A bomb or vessel in which the combustible charges can be burned. A bomb calorimeter operates at constant volume and is particularly useful for measuring energies of combustion. Four essential parts are required in any bomb calorimeter: Constant volume calorimetry, also know as bomb calorimetry, is used to measure the heat of a reaction while holding volume constant and. In. Bomb Calorimeter Constant Volume.
From www.slideserve.com
PPT Reaction carried out under constant volume. Use a bomb Bomb Calorimeter Constant Volume A bucket or container for holding the bomb in a. Four critical parts are needed in every bomb calorimeter. Constant volume calorimetry, also know as bomb calorimetry, is used to measure the heat of a reaction while holding volume constant and. A bomb calorimeter operates at constant volume and is particularly useful for measuring energies of combustion. Four essential parts. Bomb Calorimeter Constant Volume.
From www.pathwaystochemistry.com
Calorimetry Pathways to Chemistry Bomb Calorimeter Constant Volume Constant volume calorimetry, also know as bomb calorimetry, is used to measure the heat of a reaction while holding volume constant and. A bucket or container for holding the bomb in a. A bomb calorimeter operates at constant volume and is particularly useful for measuring energies of combustion. Constant volume calorimetry, also know as bomb calorimetry, is used to measure. Bomb Calorimeter Constant Volume.
From grade12uchemistry.weebly.com
Calorimetry Grade12UChemistry Bomb Calorimeter Constant Volume A bomb calorimeter operates at constant volume and is particularly useful for measuring energies of combustion. Constant volume calorimetry, also know as bomb calorimetry, is used to measure the heat of a reaction while holding volume constant and resisting large. A bucket or container for holding the bomb in a. Four essential parts are required in any bomb calorimeter: Constant. Bomb Calorimeter Constant Volume.
From www.shutterstock.com
Bomb Calorimeter Vector Illustration Labeled Educational Stock Vector Bomb Calorimeter Constant Volume In chemistry, the changes of heat of a reaction can be measured at fixed pressure or volume. Four critical parts are needed in every bomb calorimeter. A bomb or vessel in which the combustible charges can be burned. Four essential parts are required in any bomb calorimeter: Constant volume calorimetry, also know as bomb calorimetry, is used to measure the. Bomb Calorimeter Constant Volume.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation, free download ID6133898 Bomb Calorimeter Constant Volume In chemistry, the changes of heat of a reaction can be measured at fixed pressure or volume. Constant volume calorimetry, also know as bomb calorimetry, is used to measure the heat of a reaction while holding volume constant and resisting large. Four essential parts are required in any bomb calorimeter: A bucket or container for holding the bomb in a.. Bomb Calorimeter Constant Volume.
From www.chegg.com
Solved A bomb calorimeter, or a constant volume calorimeter, Bomb Calorimeter Constant Volume In chemistry, the changes of heat of a reaction can be measured at fixed pressure or volume. Four essential parts are required in any bomb calorimeter: A bomb or vessel in which the combustible charges can be burned. Four critical parts are needed in every bomb calorimeter. A bomb calorimeter operates at constant volume and is particularly useful for measuring. Bomb Calorimeter Constant Volume.
From www.youtube.com
Thermochemistry ConstantVolume Calorimeter (Bomb Calorimeter). YouTube Bomb Calorimeter Constant Volume Four critical parts are needed in every bomb calorimeter. Constant volume calorimetry, also know as bomb calorimetry, is used to measure the heat of a reaction while holding volume constant and resisting large. A bomb calorimeter operates at constant volume and is particularly useful for measuring energies of combustion. Constant volume calorimetry, also know as bomb calorimetry, is used to. Bomb Calorimeter Constant Volume.
From www.slideserve.com
PPT Ch. 6 & 8.8 lecture notes PowerPoint Presentation, free download Bomb Calorimeter Constant Volume Four critical parts are needed in every bomb calorimeter. A bucket or container for holding the bomb in a. A bomb calorimeter operates at constant volume and is particularly useful for measuring energies of combustion. Four essential parts are required in any bomb calorimeter: Constant volume calorimetry, also know as bomb calorimetry, is used to measure the heat of a. Bomb Calorimeter Constant Volume.
From www.youtube.com
Thermochemistry // Bomb Calorimeter ∆E at constant volume YouTube Bomb Calorimeter Constant Volume A bucket or container for holding the bomb in a. Four essential parts are required in any bomb calorimeter: Four critical parts are needed in every bomb calorimeter. Constant volume calorimetry, also know as bomb calorimetry, is used to measure the heat of a reaction while holding volume constant and. Constant volume calorimetry, also know as bomb calorimetry, is used. Bomb Calorimeter Constant Volume.
From www.youtube.com
CHEMISTRY 101 Calculating Heat Capacity of a Bomb Calorimeter YouTube Bomb Calorimeter Constant Volume Constant volume calorimetry, also know as bomb calorimetry, is used to measure the heat of a reaction while holding volume constant and resisting large. Constant volume calorimetry, also know as bomb calorimetry, is used to measure the heat of a reaction while holding volume constant and. A bomb calorimeter operates at constant volume and is particularly useful for measuring energies. Bomb Calorimeter Constant Volume.
From www.youtube.com
Calorimetry, Bomb Calorimetry, Constant Pressure Calorimetry FULL Bomb Calorimeter Constant Volume In chemistry, the changes of heat of a reaction can be measured at fixed pressure or volume. A bucket or container for holding the bomb in a. Four critical parts are needed in every bomb calorimeter. A bomb or vessel in which the combustible charges can be burned. A bomb calorimeter operates at constant volume and is particularly useful for. Bomb Calorimeter Constant Volume.
From www.chegg.com
Solved A bomb calorimeter, or constant volume calorimeter, Bomb Calorimeter Constant Volume Constant volume calorimetry, also know as bomb calorimetry, is used to measure the heat of a reaction while holding volume constant and resisting large. A bomb or vessel in which the combustible charges can be burned. Four essential parts are required in any bomb calorimeter: Constant volume calorimetry, also know as bomb calorimetry, is used to measure the heat of. Bomb Calorimeter Constant Volume.
From www.thoughtco.com
Calorimeter Definition in Chemistry Bomb Calorimeter Constant Volume A bucket or container for holding the bomb in a. Four critical parts are needed in every bomb calorimeter. A bomb calorimeter operates at constant volume and is particularly useful for measuring energies of combustion. Four essential parts are required in any bomb calorimeter: In chemistry, the changes of heat of a reaction can be measured at fixed pressure or. Bomb Calorimeter Constant Volume.
From www.slideserve.com
PPT Thermochemistry PowerPoint Presentation, free download ID6595728 Bomb Calorimeter Constant Volume Constant volume calorimetry, also know as bomb calorimetry, is used to measure the heat of a reaction while holding volume constant and resisting large. Four essential parts are required in any bomb calorimeter: A bucket or container for holding the bomb in a. A bomb calorimeter operates at constant volume and is particularly useful for measuring energies of combustion. Constant. Bomb Calorimeter Constant Volume.
From study.com
Bomb Calorimeter Uses, Equations & Examples Lesson Bomb Calorimeter Constant Volume A bucket or container for holding the bomb in a. A bomb calorimeter operates at constant volume and is particularly useful for measuring energies of combustion. Constant volume calorimetry, also know as bomb calorimetry, is used to measure the heat of a reaction while holding volume constant and resisting large. Constant volume calorimetry, also know as bomb calorimetry, is used. Bomb Calorimeter Constant Volume.
From www.britannica.com
Calorimeter Definition, Uses, Diagram, & Facts Britannica Bomb Calorimeter Constant Volume Four essential parts are required in any bomb calorimeter: In chemistry, the changes of heat of a reaction can be measured at fixed pressure or volume. Constant volume calorimetry, also know as bomb calorimetry, is used to measure the heat of a reaction while holding volume constant and. A bomb or vessel in which the combustible charges can be burned.. Bomb Calorimeter Constant Volume.
From www.chegg.com
Solved A bomb calorimeter, or constant volume calorimeter, Bomb Calorimeter Constant Volume Four essential parts are required in any bomb calorimeter: Constant volume calorimetry, also know as bomb calorimetry, is used to measure the heat of a reaction while holding volume constant and resisting large. Four critical parts are needed in every bomb calorimeter. A bucket or container for holding the bomb in a. Constant volume calorimetry, also know as bomb calorimetry,. Bomb Calorimeter Constant Volume.
From www.youtube.com
CHEMISTRY 101 Constant volume calorimetry YouTube Bomb Calorimeter Constant Volume Constant volume calorimetry, also know as bomb calorimetry, is used to measure the heat of a reaction while holding volume constant and resisting large. A bomb or vessel in which the combustible charges can be burned. Four critical parts are needed in every bomb calorimeter. Four essential parts are required in any bomb calorimeter: A bomb calorimeter operates at constant. Bomb Calorimeter Constant Volume.
From martinfersbanks.blogspot.com
Is a Bomb Calorimeter Constant Pressure Bomb Calorimeter Constant Volume A bucket or container for holding the bomb in a. Constant volume calorimetry, also know as bomb calorimetry, is used to measure the heat of a reaction while holding volume constant and. In chemistry, the changes of heat of a reaction can be measured at fixed pressure or volume. Four critical parts are needed in every bomb calorimeter. Four essential. Bomb Calorimeter Constant Volume.
From www.youtube.com
Bomb Calorimeter vs Coffee Cup Calorimeter Problem Constant Pressure Bomb Calorimeter Constant Volume Constant volume calorimetry, also know as bomb calorimetry, is used to measure the heat of a reaction while holding volume constant and resisting large. Constant volume calorimetry, also know as bomb calorimetry, is used to measure the heat of a reaction while holding volume constant and. A bucket or container for holding the bomb in a. Four critical parts are. Bomb Calorimeter Constant Volume.
From www.slideserve.com
PPT Thermochemistry PowerPoint Presentation, free download ID5194704 Bomb Calorimeter Constant Volume A bomb or vessel in which the combustible charges can be burned. A bucket or container for holding the bomb in a. In chemistry, the changes of heat of a reaction can be measured at fixed pressure or volume. Four essential parts are required in any bomb calorimeter: Four critical parts are needed in every bomb calorimeter. Constant volume calorimetry,. Bomb Calorimeter Constant Volume.