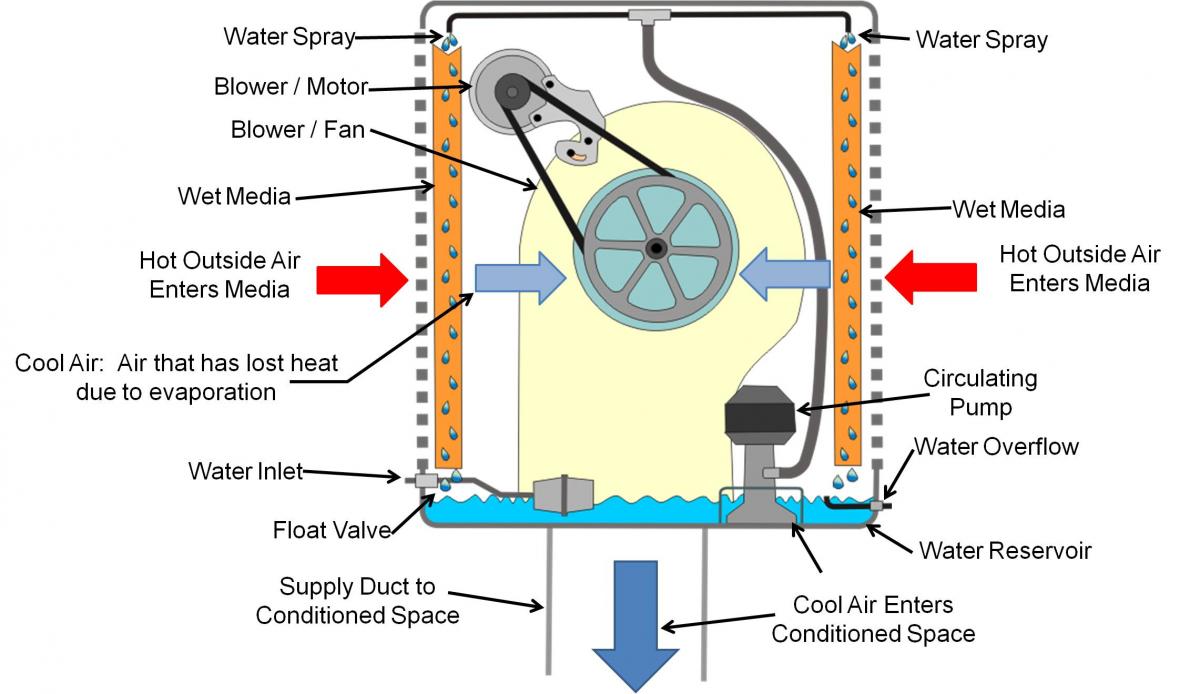
How Does Evaporative Cooling Work Physics . This energy is taken from our body, or sweat, in the. You feel cooler when you get out of a. The molecule sucks energy from surrounding molecules when it goes from a liquid to a gas causing the surrounding particles to lose. When warm, dry and unsaturated air is pulled. Evaporative cooling works on the principle that turning water to a gas requires energy. Turning a liquid such as sweat from its liquid state into a gas requires energy. This energy is taken from the air as heat and the process thereby cools the air as it extracts. The key principle for evaporative cooling is that the phase change from a liquid to a gas absorbs energy from the surroundings. It works for all liquids changing into vapor, but water is especially effective because of its high heat of vaporization.
from basc.pnnl.gov
Turning a liquid such as sweat from its liquid state into a gas requires energy. This energy is taken from the air as heat and the process thereby cools the air as it extracts. The key principle for evaporative cooling is that the phase change from a liquid to a gas absorbs energy from the surroundings. Evaporative cooling works on the principle that turning water to a gas requires energy. It works for all liquids changing into vapor, but water is especially effective because of its high heat of vaporization. The molecule sucks energy from surrounding molecules when it goes from a liquid to a gas causing the surrounding particles to lose. When warm, dry and unsaturated air is pulled. You feel cooler when you get out of a. This energy is taken from our body, or sweat, in the.
Evaporative Cooling Systems Building America Solution Center
How Does Evaporative Cooling Work Physics It works for all liquids changing into vapor, but water is especially effective because of its high heat of vaporization. Evaporative cooling works on the principle that turning water to a gas requires energy. This energy is taken from our body, or sweat, in the. It works for all liquids changing into vapor, but water is especially effective because of its high heat of vaporization. You feel cooler when you get out of a. Turning a liquid such as sweat from its liquid state into a gas requires energy. When warm, dry and unsaturated air is pulled. This energy is taken from the air as heat and the process thereby cools the air as it extracts. The key principle for evaporative cooling is that the phase change from a liquid to a gas absorbs energy from the surroundings. The molecule sucks energy from surrounding molecules when it goes from a liquid to a gas causing the surrounding particles to lose.
From design.udlvirtual.edu.pe
Working Principle Of Evaporative Cooling System Design Talk How Does Evaporative Cooling Work Physics Evaporative cooling works on the principle that turning water to a gas requires energy. It works for all liquids changing into vapor, but water is especially effective because of its high heat of vaporization. The molecule sucks energy from surrounding molecules when it goes from a liquid to a gas causing the surrounding particles to lose. When warm, dry and. How Does Evaporative Cooling Work Physics.
From studylib.net
How does an Evaporative Air Cooler work? How Does Evaporative Cooling Work Physics When warm, dry and unsaturated air is pulled. This energy is taken from our body, or sweat, in the. Turning a liquid such as sweat from its liquid state into a gas requires energy. The molecule sucks energy from surrounding molecules when it goes from a liquid to a gas causing the surrounding particles to lose. The key principle for. How Does Evaporative Cooling Work Physics.
From basc.pnnl.gov
Evaporative Cooling Systems Building America Solution Center How Does Evaporative Cooling Work Physics The key principle for evaporative cooling is that the phase change from a liquid to a gas absorbs energy from the surroundings. When warm, dry and unsaturated air is pulled. Turning a liquid such as sweat from its liquid state into a gas requires energy. It works for all liquids changing into vapor, but water is especially effective because of. How Does Evaporative Cooling Work Physics.
From www.evapco.eu
Evaporative Cooling 101 EVAPCO Europe How Does Evaporative Cooling Work Physics Evaporative cooling works on the principle that turning water to a gas requires energy. The key principle for evaporative cooling is that the phase change from a liquid to a gas absorbs energy from the surroundings. When warm, dry and unsaturated air is pulled. This energy is taken from the air as heat and the process thereby cools the air. How Does Evaporative Cooling Work Physics.
From sciencenotes.org
Evaporative Cooler How It Works and Examples How Does Evaporative Cooling Work Physics Evaporative cooling works on the principle that turning water to a gas requires energy. This energy is taken from the air as heat and the process thereby cools the air as it extracts. It works for all liquids changing into vapor, but water is especially effective because of its high heat of vaporization. You feel cooler when you get out. How Does Evaporative Cooling Work Physics.
From www.ecocooling.co.uk
How Does Evaporative Cooling Work? EcoCooling How Does Evaporative Cooling Work Physics This energy is taken from our body, or sweat, in the. This energy is taken from the air as heat and the process thereby cools the air as it extracts. You feel cooler when you get out of a. The key principle for evaporative cooling is that the phase change from a liquid to a gas absorbs energy from the. How Does Evaporative Cooling Work Physics.
From www.youtube.com
What is Evaporative Cooling and How Does it Work? YouTube How Does Evaporative Cooling Work Physics Turning a liquid such as sweat from its liquid state into a gas requires energy. This energy is taken from our body, or sweat, in the. The molecule sucks energy from surrounding molecules when it goes from a liquid to a gas causing the surrounding particles to lose. It works for all liquids changing into vapor, but water is especially. How Does Evaporative Cooling Work Physics.
From basc.pnnl.gov
Evaporative Cooling Systems Building America Solution Center How Does Evaporative Cooling Work Physics You feel cooler when you get out of a. This energy is taken from the air as heat and the process thereby cools the air as it extracts. Turning a liquid such as sweat from its liquid state into a gas requires energy. It works for all liquids changing into vapor, but water is especially effective because of its high. How Does Evaporative Cooling Work Physics.
From www.ecologycenter.us
What is an Evaporative Air Cooler and How does It different from an Air How Does Evaporative Cooling Work Physics This energy is taken from our body, or sweat, in the. This energy is taken from the air as heat and the process thereby cools the air as it extracts. You feel cooler when you get out of a. The key principle for evaporative cooling is that the phase change from a liquid to a gas absorbs energy from the. How Does Evaporative Cooling Work Physics.
From efficiencymatrix.com.au
Evaporative Cooling How Does Evaporative Cooling Work Physics The key principle for evaporative cooling is that the phase change from a liquid to a gas absorbs energy from the surroundings. It works for all liquids changing into vapor, but water is especially effective because of its high heat of vaporization. Turning a liquid such as sweat from its liquid state into a gas requires energy. When warm, dry. How Does Evaporative Cooling Work Physics.
From rulinghomes.com
How Does An Evaporative Cooler Work? Guide] How Does Evaporative Cooling Work Physics Evaporative cooling works on the principle that turning water to a gas requires energy. Turning a liquid such as sweat from its liquid state into a gas requires energy. This energy is taken from our body, or sweat, in the. It works for all liquids changing into vapor, but water is especially effective because of its high heat of vaporization.. How Does Evaporative Cooling Work Physics.
From ar.inspiredpencil.com
Evaporative Cooler Diagram How Does Evaporative Cooling Work Physics The key principle for evaporative cooling is that the phase change from a liquid to a gas absorbs energy from the surroundings. It works for all liquids changing into vapor, but water is especially effective because of its high heat of vaporization. The molecule sucks energy from surrounding molecules when it goes from a liquid to a gas causing the. How Does Evaporative Cooling Work Physics.
From www.youtube.com
evaporative cooling explained YouTube How Does Evaporative Cooling Work Physics It works for all liquids changing into vapor, but water is especially effective because of its high heat of vaporization. Evaporative cooling works on the principle that turning water to a gas requires energy. The key principle for evaporative cooling is that the phase change from a liquid to a gas absorbs energy from the surroundings. This energy is taken. How Does Evaporative Cooling Work Physics.
From rulinghomes.com
How Does An Evaporative Cooler Work? Guide] How Does Evaporative Cooling Work Physics You feel cooler when you get out of a. The key principle for evaporative cooling is that the phase change from a liquid to a gas absorbs energy from the surroundings. It works for all liquids changing into vapor, but water is especially effective because of its high heat of vaporization. This energy is taken from the air as heat. How Does Evaporative Cooling Work Physics.
From brainly.in
how does evaporation cause cooling?? Brainly.in How Does Evaporative Cooling Work Physics Evaporative cooling works on the principle that turning water to a gas requires energy. When warm, dry and unsaturated air is pulled. You feel cooler when you get out of a. This energy is taken from our body, or sweat, in the. The molecule sucks energy from surrounding molecules when it goes from a liquid to a gas causing the. How Does Evaporative Cooling Work Physics.
From design.udlvirtual.edu.pe
Explain How Evaporative Cooling Works Design Talk How Does Evaporative Cooling Work Physics The molecule sucks energy from surrounding molecules when it goes from a liquid to a gas causing the surrounding particles to lose. Turning a liquid such as sweat from its liquid state into a gas requires energy. It works for all liquids changing into vapor, but water is especially effective because of its high heat of vaporization. This energy is. How Does Evaporative Cooling Work Physics.
From fastlovehomes.com.au
Split System vs Evaporative Cooling What is the difference? How Does Evaporative Cooling Work Physics Turning a liquid such as sweat from its liquid state into a gas requires energy. This energy is taken from the air as heat and the process thereby cools the air as it extracts. Evaporative cooling works on the principle that turning water to a gas requires energy. The molecule sucks energy from surrounding molecules when it goes from a. How Does Evaporative Cooling Work Physics.
From alturascontractors.com
Evaporative Cooling Vs Refrigerated Cooling Alturas Contractors How Does Evaporative Cooling Work Physics The molecule sucks energy from surrounding molecules when it goes from a liquid to a gas causing the surrounding particles to lose. Turning a liquid such as sweat from its liquid state into a gas requires energy. This energy is taken from the air as heat and the process thereby cools the air as it extracts. It works for all. How Does Evaporative Cooling Work Physics.
From wirelealdudcarbineers.z21.web.core.windows.net
Evaporative Cooler Schematic How Does Evaporative Cooling Work Physics It works for all liquids changing into vapor, but water is especially effective because of its high heat of vaporization. Turning a liquid such as sweat from its liquid state into a gas requires energy. Evaporative cooling works on the principle that turning water to a gas requires energy. This energy is taken from our body, or sweat, in the.. How Does Evaporative Cooling Work Physics.
From www.youtube.com
Evaporative Cooling Explained YouTube How Does Evaporative Cooling Work Physics This energy is taken from our body, or sweat, in the. Turning a liquid such as sweat from its liquid state into a gas requires energy. It works for all liquids changing into vapor, but water is especially effective because of its high heat of vaporization. The molecule sucks energy from surrounding molecules when it goes from a liquid to. How Does Evaporative Cooling Work Physics.
From www.teachoo.com
How does Evaporation cause cooling? Explain (with Examples) Teachoo How Does Evaporative Cooling Work Physics This energy is taken from the air as heat and the process thereby cools the air as it extracts. Evaporative cooling works on the principle that turning water to a gas requires energy. Turning a liquid such as sweat from its liquid state into a gas requires energy. The molecule sucks energy from surrounding molecules when it goes from a. How Does Evaporative Cooling Work Physics.
From www.araner.com
What is an evaporative cooling system and how does it work? How Does Evaporative Cooling Work Physics You feel cooler when you get out of a. It works for all liquids changing into vapor, but water is especially effective because of its high heat of vaporization. Turning a liquid such as sweat from its liquid state into a gas requires energy. The molecule sucks energy from surrounding molecules when it goes from a liquid to a gas. How Does Evaporative Cooling Work Physics.
From loricchandlero.blob.core.windows.net
How Do Evaporative Cooling Systems Work at loricchandlero blog How Does Evaporative Cooling Work Physics The key principle for evaporative cooling is that the phase change from a liquid to a gas absorbs energy from the surroundings. It works for all liquids changing into vapor, but water is especially effective because of its high heat of vaporization. The molecule sucks energy from surrounding molecules when it goes from a liquid to a gas causing the. How Does Evaporative Cooling Work Physics.
From www.airwatergas.com.au
So what is Evaporative cooling and how does it work? How Does Evaporative Cooling Work Physics It works for all liquids changing into vapor, but water is especially effective because of its high heat of vaporization. This energy is taken from the air as heat and the process thereby cools the air as it extracts. When warm, dry and unsaturated air is pulled. You feel cooler when you get out of a. This energy is taken. How Does Evaporative Cooling Work Physics.
From hvaclifehack.com
How Do Evaporative Coolers Work? Here's the Answer! How Does Evaporative Cooling Work Physics Turning a liquid such as sweat from its liquid state into a gas requires energy. When warm, dry and unsaturated air is pulled. Evaporative cooling works on the principle that turning water to a gas requires energy. This energy is taken from the air as heat and the process thereby cools the air as it extracts. The key principle for. How Does Evaporative Cooling Work Physics.
From studylib.net
Evaporative Cooling Systems How and Why They Work How Does Evaporative Cooling Work Physics This energy is taken from our body, or sweat, in the. Turning a liquid such as sweat from its liquid state into a gas requires energy. The key principle for evaporative cooling is that the phase change from a liquid to a gas absorbs energy from the surroundings. It works for all liquids changing into vapor, but water is especially. How Does Evaporative Cooling Work Physics.
From mepacademy.com
How do Evaporative Coolers Work MEP Academy How Does Evaporative Cooling Work Physics Evaporative cooling works on the principle that turning water to a gas requires energy. This energy is taken from the air as heat and the process thereby cools the air as it extracts. Turning a liquid such as sweat from its liquid state into a gas requires energy. When warm, dry and unsaturated air is pulled. This energy is taken. How Does Evaporative Cooling Work Physics.
From www.powrmatic.co.uk
How Does Evaporative Cooling Work? Powrmatic How Does Evaporative Cooling Work Physics The molecule sucks energy from surrounding molecules when it goes from a liquid to a gas causing the surrounding particles to lose. This energy is taken from our body, or sweat, in the. You feel cooler when you get out of a. When warm, dry and unsaturated air is pulled. It works for all liquids changing into vapor, but water. How Does Evaporative Cooling Work Physics.
From www.slideserve.com
PPT What is evaporative cooling and how does it work PowerPoint How Does Evaporative Cooling Work Physics This energy is taken from the air as heat and the process thereby cools the air as it extracts. You feel cooler when you get out of a. When warm, dry and unsaturated air is pulled. It works for all liquids changing into vapor, but water is especially effective because of its high heat of vaporization. The molecule sucks energy. How Does Evaporative Cooling Work Physics.
From www.airwatergas.com.au
So what is Evaporative cooling and how does it work? How Does Evaporative Cooling Work Physics This energy is taken from the air as heat and the process thereby cools the air as it extracts. Turning a liquid such as sweat from its liquid state into a gas requires energy. The molecule sucks energy from surrounding molecules when it goes from a liquid to a gas causing the surrounding particles to lose. You feel cooler when. How Does Evaporative Cooling Work Physics.
From www.baysidecomfortsolutions.com.au
How Evaporative Cooling Works Bayside Comfort Solutions Heating How Does Evaporative Cooling Work Physics This energy is taken from our body, or sweat, in the. The key principle for evaporative cooling is that the phase change from a liquid to a gas absorbs energy from the surroundings. When warm, dry and unsaturated air is pulled. This energy is taken from the air as heat and the process thereby cools the air as it extracts.. How Does Evaporative Cooling Work Physics.
From basc.pnnl.gov
Evaporative Cooling Systems Building America Solution Center How Does Evaporative Cooling Work Physics The molecule sucks energy from surrounding molecules when it goes from a liquid to a gas causing the surrounding particles to lose. Evaporative cooling works on the principle that turning water to a gas requires energy. This energy is taken from our body, or sweat, in the. This energy is taken from the air as heat and the process thereby. How Does Evaporative Cooling Work Physics.
From www.therma.com
Evaporative Cooling 101 An Introduction Therma How Does Evaporative Cooling Work Physics This energy is taken from the air as heat and the process thereby cools the air as it extracts. Turning a liquid such as sweat from its liquid state into a gas requires energy. It works for all liquids changing into vapor, but water is especially effective because of its high heat of vaporization. The molecule sucks energy from surrounding. How Does Evaporative Cooling Work Physics.
From www.thespruce.com
The Parts of an Evaporative Cooler (Swamp Cooler) How Does Evaporative Cooling Work Physics You feel cooler when you get out of a. This energy is taken from our body, or sweat, in the. This energy is taken from the air as heat and the process thereby cools the air as it extracts. The molecule sucks energy from surrounding molecules when it goes from a liquid to a gas causing the surrounding particles to. How Does Evaporative Cooling Work Physics.
From portacool.com
How Does Evaporative Cooling Work? Portacool How Does Evaporative Cooling Work Physics It works for all liquids changing into vapor, but water is especially effective because of its high heat of vaporization. The molecule sucks energy from surrounding molecules when it goes from a liquid to a gas causing the surrounding particles to lose. When warm, dry and unsaturated air is pulled. This energy is taken from the air as heat and. How Does Evaporative Cooling Work Physics.