Electrodes For Electrolysis Cell . Here, the redox reaction is spontaneous and is responsible for the production. An electrolytic cell is in this sense. These cells are called electrolytic cells. Perform stoichiometric calculations for electrolytic. An electrolytic cell converts electrical energy into chemical energy. The electrode of an electrochemical cell at which. Describe the process of electrolysis. There are two electrodes, and the battery transfers electrons between the cathode , where reduction takes place, and the anode , where oxidation takes place. The locations where the chemical reaction occurs are called electrodes. Compare the operation of electrolytic cells with that of galvanic cells. In any electrochemical cell (electrolytic or galvanic) the electrode at which reduction occurs is called the cathode. The electrolysis of molten nacl. The chemical change produced by passing an electric current through a conducting solution or a molten salt.
from fixlibrarybaladinas3c.z4.web.core.windows.net
The electrode of an electrochemical cell at which. Compare the operation of electrolytic cells with that of galvanic cells. The chemical change produced by passing an electric current through a conducting solution or a molten salt. Describe the process of electrolysis. An electrolytic cell is in this sense. An electrolytic cell converts electrical energy into chemical energy. The locations where the chemical reaction occurs are called electrodes. Here, the redox reaction is spontaneous and is responsible for the production. These cells are called electrolytic cells. There are two electrodes, and the battery transfers electrons between the cathode , where reduction takes place, and the anode , where oxidation takes place.
What Happens At The Cathode In Electrolysis
Electrodes For Electrolysis Cell Perform stoichiometric calculations for electrolytic. Compare the operation of electrolytic cells with that of galvanic cells. The electrode of an electrochemical cell at which. An electrolytic cell converts electrical energy into chemical energy. Here, the redox reaction is spontaneous and is responsible for the production. The locations where the chemical reaction occurs are called electrodes. In any electrochemical cell (electrolytic or galvanic) the electrode at which reduction occurs is called the cathode. The chemical change produced by passing an electric current through a conducting solution or a molten salt. These cells are called electrolytic cells. There are two electrodes, and the battery transfers electrons between the cathode , where reduction takes place, and the anode , where oxidation takes place. Perform stoichiometric calculations for electrolytic. The electrolysis of molten nacl. Describe the process of electrolysis. An electrolytic cell is in this sense.
From www.researchgate.net
Schematic illustration of a typical three‐electrode system. Download Scientific Diagram Electrodes For Electrolysis Cell An electrolytic cell is in this sense. Here, the redox reaction is spontaneous and is responsible for the production. The electrode of an electrochemical cell at which. The electrolysis of molten nacl. The chemical change produced by passing an electric current through a conducting solution or a molten salt. An electrolytic cell converts electrical energy into chemical energy. There are. Electrodes For Electrolysis Cell.
From www.teachoo.com
Electrolytic Cell Definition, Components, Examples Teachoo Electrodes For Electrolysis Cell In any electrochemical cell (electrolytic or galvanic) the electrode at which reduction occurs is called the cathode. An electrolytic cell converts electrical energy into chemical energy. Compare the operation of electrolytic cells with that of galvanic cells. The chemical change produced by passing an electric current through a conducting solution or a molten salt. Here, the redox reaction is spontaneous. Electrodes For Electrolysis Cell.
From www.chemicals.co.uk
A Level Chemistry Electrodes & Electrochemical Cells Electrodes For Electrolysis Cell Perform stoichiometric calculations for electrolytic. The locations where the chemical reaction occurs are called electrodes. An electrolytic cell converts electrical energy into chemical energy. The chemical change produced by passing an electric current through a conducting solution or a molten salt. Compare the operation of electrolytic cells with that of galvanic cells. The electrolysis of molten nacl. Here, the redox. Electrodes For Electrolysis Cell.
From stock.adobe.com
Electrolytic cell infographic diagram with components including anode cathode electrolyte Electrodes For Electrolysis Cell There are two electrodes, and the battery transfers electrons between the cathode , where reduction takes place, and the anode , where oxidation takes place. Compare the operation of electrolytic cells with that of galvanic cells. Perform stoichiometric calculations for electrolytic. The chemical change produced by passing an electric current through a conducting solution or a molten salt. The electrode. Electrodes For Electrolysis Cell.
From fixlibrarygedwaaldebx.z21.web.core.windows.net
Cathode Electrolyte Circuit Diagram Electrodes For Electrolysis Cell Compare the operation of electrolytic cells with that of galvanic cells. The electrolysis of molten nacl. The locations where the chemical reaction occurs are called electrodes. An electrolytic cell converts electrical energy into chemical energy. There are two electrodes, and the battery transfers electrons between the cathode , where reduction takes place, and the anode , where oxidation takes place.. Electrodes For Electrolysis Cell.
From chem.libretexts.org
Chapter 19.1 Describing Electrochemical Cells Chemistry LibreTexts Electrodes For Electrolysis Cell There are two electrodes, and the battery transfers electrons between the cathode , where reduction takes place, and the anode , where oxidation takes place. The chemical change produced by passing an electric current through a conducting solution or a molten salt. An electrolytic cell converts electrical energy into chemical energy. These cells are called electrolytic cells. The locations where. Electrodes For Electrolysis Cell.
From www.revisechemistry.uk
Electrolysis OCR Gateway C3 revisechemistry.uk Electrodes For Electrolysis Cell In any electrochemical cell (electrolytic or galvanic) the electrode at which reduction occurs is called the cathode. The chemical change produced by passing an electric current through a conducting solution or a molten salt. The electrode of an electrochemical cell at which. Describe the process of electrolysis. The locations where the chemical reaction occurs are called electrodes. Compare the operation. Electrodes For Electrolysis Cell.
From www.researchgate.net
Scheme of an experimental 3 electrode electrochemical cell. Download Scientific Diagram Electrodes For Electrolysis Cell Perform stoichiometric calculations for electrolytic. The chemical change produced by passing an electric current through a conducting solution or a molten salt. An electrolytic cell converts electrical energy into chemical energy. Here, the redox reaction is spontaneous and is responsible for the production. The electrode of an electrochemical cell at which. The locations where the chemical reaction occurs are called. Electrodes For Electrolysis Cell.
From classnotes.org.in
Electrolytic Cells Chemistry, Class 12, Electro Chemistry Electrodes For Electrolysis Cell The electrolysis of molten nacl. The chemical change produced by passing an electric current through a conducting solution or a molten salt. Perform stoichiometric calculations for electrolytic. Compare the operation of electrolytic cells with that of galvanic cells. Here, the redox reaction is spontaneous and is responsible for the production. These cells are called electrolytic cells. An electrolytic cell converts. Electrodes For Electrolysis Cell.
From keyesvannuyshyundai.blogspot.com
draw the diagram of electrolytic cell and explain keyesvannuyshyundai Electrodes For Electrolysis Cell These cells are called electrolytic cells. The chemical change produced by passing an electric current through a conducting solution or a molten salt. There are two electrodes, and the battery transfers electrons between the cathode , where reduction takes place, and the anode , where oxidation takes place. An electrolytic cell converts electrical energy into chemical energy. Perform stoichiometric calculations. Electrodes For Electrolysis Cell.
From www.teacharesources.com
Grade 12 A Science Galvanic and electrolytic cells, electrode potentials and redox in Electrodes For Electrolysis Cell The locations where the chemical reaction occurs are called electrodes. Describe the process of electrolysis. Perform stoichiometric calculations for electrolytic. The chemical change produced by passing an electric current through a conducting solution or a molten salt. Here, the redox reaction is spontaneous and is responsible for the production. Compare the operation of electrolytic cells with that of galvanic cells.. Electrodes For Electrolysis Cell.
From schoolbag.info
Electrochemical Cells Electrochemistry Training MCAT General Chemistry Review Electrodes For Electrolysis Cell Perform stoichiometric calculations for electrolytic. An electrolytic cell converts electrical energy into chemical energy. The locations where the chemical reaction occurs are called electrodes. Compare the operation of electrolytic cells with that of galvanic cells. The electrolysis of molten nacl. Here, the redox reaction is spontaneous and is responsible for the production. The chemical change produced by passing an electric. Electrodes For Electrolysis Cell.
From www.researchgate.net
Schematic of threeelectrode electrolytic cell, used in experiments.... Download Scientific Electrodes For Electrolysis Cell The electrolysis of molten nacl. There are two electrodes, and the battery transfers electrons between the cathode , where reduction takes place, and the anode , where oxidation takes place. Compare the operation of electrolytic cells with that of galvanic cells. Perform stoichiometric calculations for electrolytic. Describe the process of electrolysis. The locations where the chemical reaction occurs are called. Electrodes For Electrolysis Cell.
From philschatz.com
Electrolysis · Chemistry Electrodes For Electrolysis Cell The chemical change produced by passing an electric current through a conducting solution or a molten salt. Here, the redox reaction is spontaneous and is responsible for the production. An electrolytic cell is in this sense. Describe the process of electrolysis. The locations where the chemical reaction occurs are called electrodes. An electrolytic cell converts electrical energy into chemical energy.. Electrodes For Electrolysis Cell.
From sciencevision.in
Electrolytes , Electolytic Cell And Electrochemical Cell Science Vision Electrodes For Electrolysis Cell Perform stoichiometric calculations for electrolytic. An electrolytic cell is in this sense. An electrolytic cell converts electrical energy into chemical energy. Compare the operation of electrolytic cells with that of galvanic cells. Describe the process of electrolysis. Here, the redox reaction is spontaneous and is responsible for the production. The electrolysis of molten nacl. The chemical change produced by passing. Electrodes For Electrolysis Cell.
From www.researchgate.net
The convention three electrode cell configuration for electrochemical... Download Scientific Electrodes For Electrolysis Cell The locations where the chemical reaction occurs are called electrodes. The chemical change produced by passing an electric current through a conducting solution or a molten salt. The electrolysis of molten nacl. In any electrochemical cell (electrolytic or galvanic) the electrode at which reduction occurs is called the cathode. Compare the operation of electrolytic cells with that of galvanic cells.. Electrodes For Electrolysis Cell.
From chem.libretexts.org
20.4 Cell Potential Under Standard Conditions Chemistry LibreTexts Electrodes For Electrolysis Cell An electrolytic cell is in this sense. In any electrochemical cell (electrolytic or galvanic) the electrode at which reduction occurs is called the cathode. The electrolysis of molten nacl. The chemical change produced by passing an electric current through a conducting solution or a molten salt. The locations where the chemical reaction occurs are called electrodes. Perform stoichiometric calculations for. Electrodes For Electrolysis Cell.
From www.researchgate.net
Schematic illustration of threeelectrode electrochemical Hcell. Download Scientific Diagram Electrodes For Electrolysis Cell The chemical change produced by passing an electric current through a conducting solution or a molten salt. Compare the operation of electrolytic cells with that of galvanic cells. An electrolytic cell is in this sense. The locations where the chemical reaction occurs are called electrodes. Describe the process of electrolysis. In any electrochemical cell (electrolytic or galvanic) the electrode at. Electrodes For Electrolysis Cell.
From www.researchgate.net
Figure The anode and cathode reactions in typical electrolytic... Download Scientific Diagram Electrodes For Electrolysis Cell An electrolytic cell converts electrical energy into chemical energy. In any electrochemical cell (electrolytic or galvanic) the electrode at which reduction occurs is called the cathode. The electrolysis of molten nacl. The electrode of an electrochemical cell at which. The chemical change produced by passing an electric current through a conducting solution or a molten salt. An electrolytic cell is. Electrodes For Electrolysis Cell.
From www.sigmaaldrich.com
Electrochemistry on the Bench and in the Field Electrodes For Electrolysis Cell Perform stoichiometric calculations for electrolytic. An electrolytic cell converts electrical energy into chemical energy. The electrolysis of molten nacl. Compare the operation of electrolytic cells with that of galvanic cells. Describe the process of electrolysis. These cells are called electrolytic cells. The electrode of an electrochemical cell at which. Here, the redox reaction is spontaneous and is responsible for the. Electrodes For Electrolysis Cell.
From spmscience.blog.onlinetuition.com.my
Electrolysis SPM Science Electrodes For Electrolysis Cell The locations where the chemical reaction occurs are called electrodes. The electrolysis of molten nacl. The electrode of an electrochemical cell at which. There are two electrodes, and the battery transfers electrons between the cathode , where reduction takes place, and the anode , where oxidation takes place. Here, the redox reaction is spontaneous and is responsible for the production.. Electrodes For Electrolysis Cell.
From alevelchemistry.co.uk
Electrochemical Cells Definition, Description & Types Electrodes For Electrolysis Cell In any electrochemical cell (electrolytic or galvanic) the electrode at which reduction occurs is called the cathode. The electrolysis of molten nacl. The chemical change produced by passing an electric current through a conducting solution or a molten salt. These cells are called electrolytic cells. An electrolytic cell is in this sense. Perform stoichiometric calculations for electrolytic. There are two. Electrodes For Electrolysis Cell.
From www.researchgate.net
Scheme of twoelectrode system for overall water splitting. HER,... Download Scientific Diagram Electrodes For Electrolysis Cell Compare the operation of electrolytic cells with that of galvanic cells. The electrode of an electrochemical cell at which. An electrolytic cell converts electrical energy into chemical energy. An electrolytic cell is in this sense. In any electrochemical cell (electrolytic or galvanic) the electrode at which reduction occurs is called the cathode. The locations where the chemical reaction occurs are. Electrodes For Electrolysis Cell.
From celwrbgp.blob.core.windows.net
Graphite Electrode Bromide at Nancy Espinosa blog Electrodes For Electrolysis Cell Perform stoichiometric calculations for electrolytic. The locations where the chemical reaction occurs are called electrodes. In any electrochemical cell (electrolytic or galvanic) the electrode at which reduction occurs is called the cathode. Here, the redox reaction is spontaneous and is responsible for the production. The electrode of an electrochemical cell at which. Compare the operation of electrolytic cells with that. Electrodes For Electrolysis Cell.
From fixlibrarybaladinas3c.z4.web.core.windows.net
What Happens At The Cathode In Electrolysis Electrodes For Electrolysis Cell An electrolytic cell converts electrical energy into chemical energy. Describe the process of electrolysis. Perform stoichiometric calculations for electrolytic. Here, the redox reaction is spontaneous and is responsible for the production. The chemical change produced by passing an electric current through a conducting solution or a molten salt. The electrode of an electrochemical cell at which. These cells are called. Electrodes For Electrolysis Cell.
From mavink.com
Electrochemical Cell Diagram Electrodes For Electrolysis Cell Compare the operation of electrolytic cells with that of galvanic cells. The electrolysis of molten nacl. Here, the redox reaction is spontaneous and is responsible for the production. The locations where the chemical reaction occurs are called electrodes. Describe the process of electrolysis. An electrolytic cell is in this sense. In any electrochemical cell (electrolytic or galvanic) the electrode at. Electrodes For Electrolysis Cell.
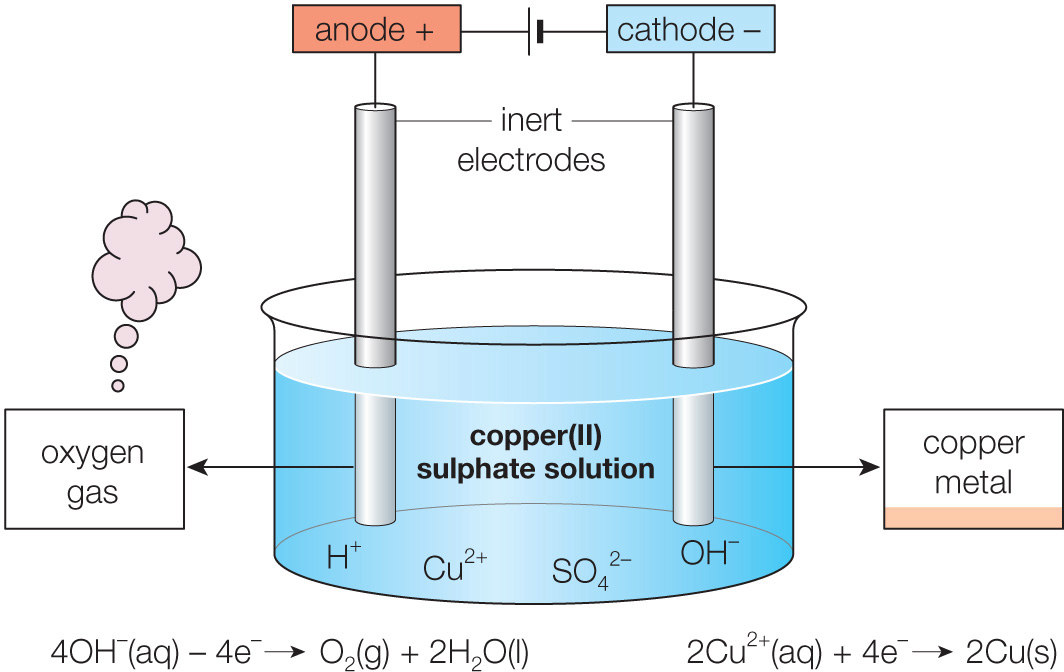
From www.alamy.com
Electroplating with copper using copper sulfate electrolyte. Electrolysis of copper(II) sulfate Electrodes For Electrolysis Cell The electrode of an electrochemical cell at which. In any electrochemical cell (electrolytic or galvanic) the electrode at which reduction occurs is called the cathode. An electrolytic cell converts electrical energy into chemical energy. The electrolysis of molten nacl. There are two electrodes, and the battery transfers electrons between the cathode , where reduction takes place, and the anode ,. Electrodes For Electrolysis Cell.
From chem.libretexts.org
19.9 Electrolysis Chemistry LibreTexts Electrodes For Electrolysis Cell There are two electrodes, and the battery transfers electrons between the cathode , where reduction takes place, and the anode , where oxidation takes place. An electrolytic cell is in this sense. In any electrochemical cell (electrolytic or galvanic) the electrode at which reduction occurs is called the cathode. The locations where the chemical reaction occurs are called electrodes. These. Electrodes For Electrolysis Cell.
From chem.libretexts.org
20.4 Cell Potential Under Standard Conditions Chemistry LibreTexts Electrodes For Electrolysis Cell Compare the operation of electrolytic cells with that of galvanic cells. Perform stoichiometric calculations for electrolytic. In any electrochemical cell (electrolytic or galvanic) the electrode at which reduction occurs is called the cathode. The electrode of an electrochemical cell at which. An electrolytic cell is in this sense. The chemical change produced by passing an electric current through a conducting. Electrodes For Electrolysis Cell.
From www.alamy.com
Electrolysis process vector illustration. Simple electrolysis process of an electrolyte Stock Electrodes For Electrolysis Cell These cells are called electrolytic cells. The chemical change produced by passing an electric current through a conducting solution or a molten salt. In any electrochemical cell (electrolytic or galvanic) the electrode at which reduction occurs is called the cathode. Perform stoichiometric calculations for electrolytic. Compare the operation of electrolytic cells with that of galvanic cells. An electrolytic cell converts. Electrodes For Electrolysis Cell.
From www.revisechemistry.uk
Electrolysis OCR Gateway C3 revisechemistry.uk Electrodes For Electrolysis Cell Perform stoichiometric calculations for electrolytic. The chemical change produced by passing an electric current through a conducting solution or a molten salt. An electrolytic cell converts electrical energy into chemical energy. In any electrochemical cell (electrolytic or galvanic) the electrode at which reduction occurs is called the cathode. These cells are called electrolytic cells. The locations where the chemical reaction. Electrodes For Electrolysis Cell.
From www.chemicals.co.uk
A Level Chemistry Electrodes & Electrochemical Cells Electrodes For Electrolysis Cell The electrode of an electrochemical cell at which. The electrolysis of molten nacl. These cells are called electrolytic cells. Here, the redox reaction is spontaneous and is responsible for the production. An electrolytic cell converts electrical energy into chemical energy. Perform stoichiometric calculations for electrolytic. There are two electrodes, and the battery transfers electrons between the cathode , where reduction. Electrodes For Electrolysis Cell.
From mungfali.com
Glass Electrode Diagram Electrodes For Electrolysis Cell Compare the operation of electrolytic cells with that of galvanic cells. The electrode of an electrochemical cell at which. The electrolysis of molten nacl. Describe the process of electrolysis. These cells are called electrolytic cells. In any electrochemical cell (electrolytic or galvanic) the electrode at which reduction occurs is called the cathode. An electrolytic cell converts electrical energy into chemical. Electrodes For Electrolysis Cell.
From chem.libretexts.org
5.6 Day 41 Electrolysis; Commercial Batteries Chemistry LibreTexts Electrodes For Electrolysis Cell An electrolytic cell is in this sense. These cells are called electrolytic cells. Describe the process of electrolysis. Here, the redox reaction is spontaneous and is responsible for the production. Compare the operation of electrolytic cells with that of galvanic cells. An electrolytic cell converts electrical energy into chemical energy. The chemical change produced by passing an electric current through. Electrodes For Electrolysis Cell.
From diagramlibrarypern.z21.web.core.windows.net
Cathode In Electrochemical Cell Electrodes For Electrolysis Cell These cells are called electrolytic cells. In any electrochemical cell (electrolytic or galvanic) the electrode at which reduction occurs is called the cathode. There are two electrodes, and the battery transfers electrons between the cathode , where reduction takes place, and the anode , where oxidation takes place. An electrolytic cell is in this sense. The electrode of an electrochemical. Electrodes For Electrolysis Cell.