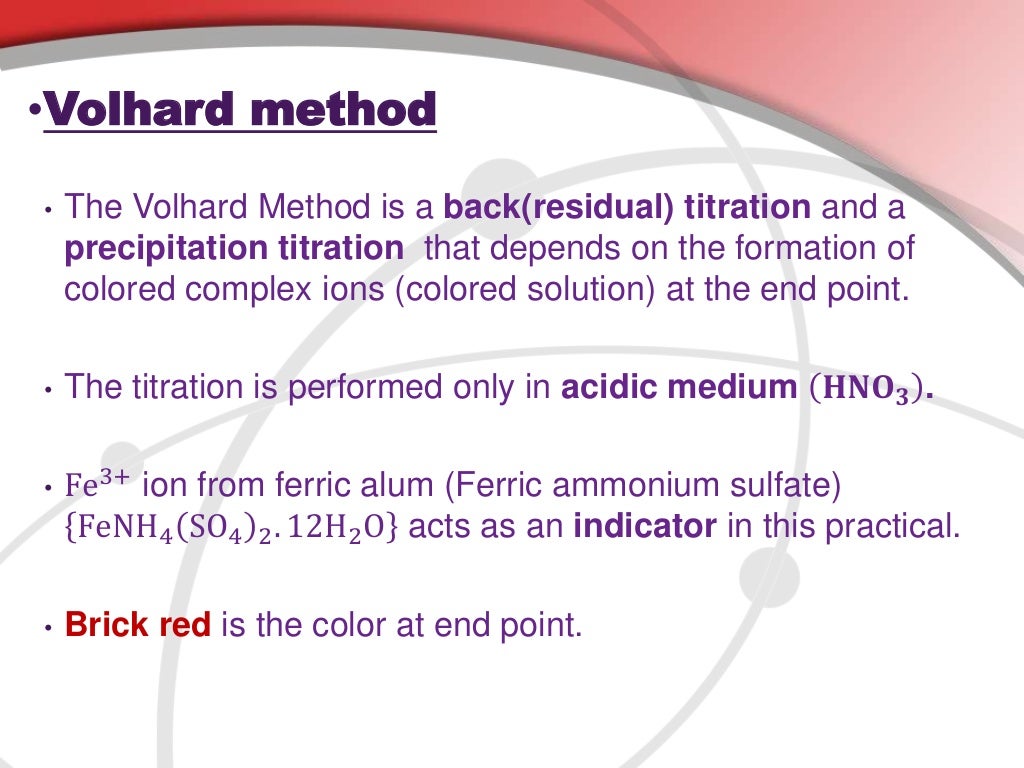
Chloride Ion Determination . The agno 3 reacts with chloride ion in a 1:1 ratio. Theory of determination of chloride in water. The concentration of chloride ions is determined by subtracting the titration findings of the moles of silver ions that reacted with the thiocyanate. A simple, direct and accurate method for chloride determination. The amount of chloride in water can be simply determined by titrating the collected water sample with silver nitrate solution by using potassium chromate indicator. In this experiment, the amount of chloride in an unknown sample was determined. This procedure will determine the concentration of chloride ion with a chloride specific ion electrode using potentiometry. Beside acid/base titrations, the titrimetric determination of chloride is one of the most frequently used titrimetric methods of analysis.
from www.slideshare.net
Theory of determination of chloride in water. This procedure will determine the concentration of chloride ion with a chloride specific ion electrode using potentiometry. In this experiment, the amount of chloride in an unknown sample was determined. Beside acid/base titrations, the titrimetric determination of chloride is one of the most frequently used titrimetric methods of analysis. The amount of chloride in water can be simply determined by titrating the collected water sample with silver nitrate solution by using potassium chromate indicator. The concentration of chloride ions is determined by subtracting the titration findings of the moles of silver ions that reacted with the thiocyanate. A simple, direct and accurate method for chloride determination. The agno 3 reacts with chloride ion in a 1:1 ratio.
Determination of chloride content in water by Volhard method
Chloride Ion Determination A simple, direct and accurate method for chloride determination. This procedure will determine the concentration of chloride ion with a chloride specific ion electrode using potentiometry. The concentration of chloride ions is determined by subtracting the titration findings of the moles of silver ions that reacted with the thiocyanate. Beside acid/base titrations, the titrimetric determination of chloride is one of the most frequently used titrimetric methods of analysis. In this experiment, the amount of chloride in an unknown sample was determined. The amount of chloride in water can be simply determined by titrating the collected water sample with silver nitrate solution by using potassium chromate indicator. A simple, direct and accurate method for chloride determination. The agno 3 reacts with chloride ion in a 1:1 ratio. Theory of determination of chloride in water.
From www.slideshare.net
Determination of chloride content in water by Volhard method Chloride Ion Determination In this experiment, the amount of chloride in an unknown sample was determined. A simple, direct and accurate method for chloride determination. Theory of determination of chloride in water. This procedure will determine the concentration of chloride ion with a chloride specific ion electrode using potentiometry. Beside acid/base titrations, the titrimetric determination of chloride is one of the most frequently. Chloride Ion Determination.
From www.studypool.com
SOLUTION Lab 7 determination of chloride Studypool Chloride Ion Determination The agno 3 reacts with chloride ion in a 1:1 ratio. In this experiment, the amount of chloride in an unknown sample was determined. The amount of chloride in water can be simply determined by titrating the collected water sample with silver nitrate solution by using potassium chromate indicator. A simple, direct and accurate method for chloride determination. The concentration. Chloride Ion Determination.
From www.yumpu.com
Determination of Chloride Ion Concentration by Chemteach Chloride Ion Determination This procedure will determine the concentration of chloride ion with a chloride specific ion electrode using potentiometry. The concentration of chloride ions is determined by subtracting the titration findings of the moles of silver ions that reacted with the thiocyanate. A simple, direct and accurate method for chloride determination. Theory of determination of chloride in water. Beside acid/base titrations, the. Chloride Ion Determination.
From www.studypool.com
SOLUTION Lab 7 determination of chloride Studypool Chloride Ion Determination The concentration of chloride ions is determined by subtracting the titration findings of the moles of silver ions that reacted with the thiocyanate. A simple, direct and accurate method for chloride determination. Theory of determination of chloride in water. The agno 3 reacts with chloride ion in a 1:1 ratio. In this experiment, the amount of chloride in an unknown. Chloride Ion Determination.
From www.slideshare.net
Determination of chloride content in water by Volhard method Chloride Ion Determination A simple, direct and accurate method for chloride determination. This procedure will determine the concentration of chloride ion with a chloride specific ion electrode using potentiometry. In this experiment, the amount of chloride in an unknown sample was determined. Theory of determination of chloride in water. The concentration of chloride ions is determined by subtracting the titration findings of the. Chloride Ion Determination.
From www.numerade.com
SOLVED Determination of Chloride Ion in Seawater by Mohr'Method Chloride Ion Determination This procedure will determine the concentration of chloride ion with a chloride specific ion electrode using potentiometry. The amount of chloride in water can be simply determined by titrating the collected water sample with silver nitrate solution by using potassium chromate indicator. The concentration of chloride ions is determined by subtracting the titration findings of the moles of silver ions. Chloride Ion Determination.
From www.researchgate.net
Determination of chloride ion content in subway service environments Chloride Ion Determination Beside acid/base titrations, the titrimetric determination of chloride is one of the most frequently used titrimetric methods of analysis. This procedure will determine the concentration of chloride ion with a chloride specific ion electrode using potentiometry. Theory of determination of chloride in water. The concentration of chloride ions is determined by subtracting the titration findings of the moles of silver. Chloride Ion Determination.
From www.youtube.com
Estimation of Chloride Ion in Water Sample in Tamil Mohr's Method Chloride Ion Determination The amount of chloride in water can be simply determined by titrating the collected water sample with silver nitrate solution by using potassium chromate indicator. This procedure will determine the concentration of chloride ion with a chloride specific ion electrode using potentiometry. In this experiment, the amount of chloride in an unknown sample was determined. A simple, direct and accurate. Chloride Ion Determination.
From www.researchgate.net
Experimental Procedures for Determination of Chloride ion... Download Chloride Ion Determination Theory of determination of chloride in water. This procedure will determine the concentration of chloride ion with a chloride specific ion electrode using potentiometry. The concentration of chloride ions is determined by subtracting the titration findings of the moles of silver ions that reacted with the thiocyanate. In this experiment, the amount of chloride in an unknown sample was determined.. Chloride Ion Determination.
From www.en-standard.eu
BS 633741984 General methods of chemical analysis Method for Chloride Ion Determination The amount of chloride in water can be simply determined by titrating the collected water sample with silver nitrate solution by using potassium chromate indicator. In this experiment, the amount of chloride in an unknown sample was determined. The agno 3 reacts with chloride ion in a 1:1 ratio. Beside acid/base titrations, the titrimetric determination of chloride is one of. Chloride Ion Determination.
From www.semanticscholar.org
Figure 1 from Coulometric Determination of Chloride Ion Using Twostep Chloride Ion Determination A simple, direct and accurate method for chloride determination. In this experiment, the amount of chloride in an unknown sample was determined. This procedure will determine the concentration of chloride ion with a chloride specific ion electrode using potentiometry. The agno 3 reacts with chloride ion in a 1:1 ratio. The concentration of chloride ions is determined by subtracting the. Chloride Ion Determination.
From www.youtube.com
Determination of chloride content in water sample by Mohr’s method Chloride Ion Determination The agno 3 reacts with chloride ion in a 1:1 ratio. In this experiment, the amount of chloride in an unknown sample was determined. The amount of chloride in water can be simply determined by titrating the collected water sample with silver nitrate solution by using potassium chromate indicator. Theory of determination of chloride in water. This procedure will determine. Chloride Ion Determination.
From docslib.org
Determination of Chloride Ion Concentration by Titration (Volhard’S Chloride Ion Determination Beside acid/base titrations, the titrimetric determination of chloride is one of the most frequently used titrimetric methods of analysis. A simple, direct and accurate method for chloride determination. The concentration of chloride ions is determined by subtracting the titration findings of the moles of silver ions that reacted with the thiocyanate. In this experiment, the amount of chloride in an. Chloride Ion Determination.
From www.researchgate.net
(PDF) DETERMINATION OF CHLORIDE CONTENT IN BOTTLED MINERAL WATER Chloride Ion Determination The amount of chloride in water can be simply determined by titrating the collected water sample with silver nitrate solution by using potassium chromate indicator. Beside acid/base titrations, the titrimetric determination of chloride is one of the most frequently used titrimetric methods of analysis. The concentration of chloride ions is determined by subtracting the titration findings of the moles of. Chloride Ion Determination.
From studylib.net
Mohr Method for Chloride Determination Chloride Ion Determination A simple, direct and accurate method for chloride determination. The amount of chloride in water can be simply determined by titrating the collected water sample with silver nitrate solution by using potassium chromate indicator. In this experiment, the amount of chloride in an unknown sample was determined. Beside acid/base titrations, the titrimetric determination of chloride is one of the most. Chloride Ion Determination.
From www.chegg.com
Solved Experiment 8 Determination of chloride ion in water Chloride Ion Determination In this experiment, the amount of chloride in an unknown sample was determined. A simple, direct and accurate method for chloride determination. The amount of chloride in water can be simply determined by titrating the collected water sample with silver nitrate solution by using potassium chromate indicator. The agno 3 reacts with chloride ion in a 1:1 ratio. Beside acid/base. Chloride Ion Determination.
From www.slideserve.com
PPT GRAVIMETRIC ESTIMATION OF CHLORIDE IONS PowerPoint Presentation Chloride Ion Determination This procedure will determine the concentration of chloride ion with a chloride specific ion electrode using potentiometry. The concentration of chloride ions is determined by subtracting the titration findings of the moles of silver ions that reacted with the thiocyanate. The amount of chloride in water can be simply determined by titrating the collected water sample with silver nitrate solution. Chloride Ion Determination.
From www.youtube.com
Determination or Assay of Sodium Chloride (NaCl) by Titration_A Chloride Ion Determination Theory of determination of chloride in water. The concentration of chloride ions is determined by subtracting the titration findings of the moles of silver ions that reacted with the thiocyanate. A simple, direct and accurate method for chloride determination. The agno 3 reacts with chloride ion in a 1:1 ratio. The amount of chloride in water can be simply determined. Chloride Ion Determination.
From studylib.net
1 Gravimetric Determination of Chloride Introduction The chloride Chloride Ion Determination In this experiment, the amount of chloride in an unknown sample was determined. The amount of chloride in water can be simply determined by titrating the collected water sample with silver nitrate solution by using potassium chromate indicator. The agno 3 reacts with chloride ion in a 1:1 ratio. Beside acid/base titrations, the titrimetric determination of chloride is one of. Chloride Ion Determination.
From www.studocu.com
Determination of Chloride ions in a given water sample Determination Chloride Ion Determination The concentration of chloride ions is determined by subtracting the titration findings of the moles of silver ions that reacted with the thiocyanate. A simple, direct and accurate method for chloride determination. Theory of determination of chloride in water. In this experiment, the amount of chloride in an unknown sample was determined. Beside acid/base titrations, the titrimetric determination of chloride. Chloride Ion Determination.
From www.chegg.com
Solved Introduction The Mohr method is used in the Chloride Ion Determination The amount of chloride in water can be simply determined by titrating the collected water sample with silver nitrate solution by using potassium chromate indicator. The concentration of chloride ions is determined by subtracting the titration findings of the moles of silver ions that reacted with the thiocyanate. In this experiment, the amount of chloride in an unknown sample was. Chloride Ion Determination.
From studylib.net
Determination of Chloride by the Mohr Method Chloride Ion Determination In this experiment, the amount of chloride in an unknown sample was determined. The agno 3 reacts with chloride ion in a 1:1 ratio. Theory of determination of chloride in water. The concentration of chloride ions is determined by subtracting the titration findings of the moles of silver ions that reacted with the thiocyanate. A simple, direct and accurate method. Chloride Ion Determination.
From www.studocu.com
Determination of Chlorides by Mohr Method Introduction The Mohr Chloride Ion Determination A simple, direct and accurate method for chloride determination. In this experiment, the amount of chloride in an unknown sample was determined. Beside acid/base titrations, the titrimetric determination of chloride is one of the most frequently used titrimetric methods of analysis. The agno 3 reacts with chloride ion in a 1:1 ratio. The concentration of chloride ions is determined by. Chloride Ion Determination.
From www.chegg.com
Solved Determination of Chloride lon in Seawater by Mohr's Chloride Ion Determination In this experiment, the amount of chloride in an unknown sample was determined. Beside acid/base titrations, the titrimetric determination of chloride is one of the most frequently used titrimetric methods of analysis. A simple, direct and accurate method for chloride determination. Theory of determination of chloride in water. The agno 3 reacts with chloride ion in a 1:1 ratio. The. Chloride Ion Determination.
From www.researchgate.net
Determination of watersoluble chloride content of concrete Download Chloride Ion Determination Theory of determination of chloride in water. A simple, direct and accurate method for chloride determination. The amount of chloride in water can be simply determined by titrating the collected water sample with silver nitrate solution by using potassium chromate indicator. In this experiment, the amount of chloride in an unknown sample was determined. This procedure will determine the concentration. Chloride Ion Determination.
From www.numerade.com
SOLVED This method determines the chloride ion concentration of a Chloride Ion Determination A simple, direct and accurate method for chloride determination. The agno 3 reacts with chloride ion in a 1:1 ratio. The amount of chloride in water can be simply determined by titrating the collected water sample with silver nitrate solution by using potassium chromate indicator. In this experiment, the amount of chloride in an unknown sample was determined. Theory of. Chloride Ion Determination.
From www.studocu.com
Determination of Chloride Ion Concentration by Titration (Mohr's Method Chloride Ion Determination The agno 3 reacts with chloride ion in a 1:1 ratio. This procedure will determine the concentration of chloride ion with a chloride specific ion electrode using potentiometry. The amount of chloride in water can be simply determined by titrating the collected water sample with silver nitrate solution by using potassium chromate indicator. Beside acid/base titrations, the titrimetric determination of. Chloride Ion Determination.
From www.youtube.com
Determination of Chloride Ions in Water Exp 4 YouTube Chloride Ion Determination The amount of chloride in water can be simply determined by titrating the collected water sample with silver nitrate solution by using potassium chromate indicator. This procedure will determine the concentration of chloride ion with a chloride specific ion electrode using potentiometry. In this experiment, the amount of chloride in an unknown sample was determined. A simple, direct and accurate. Chloride Ion Determination.
From www.researchgate.net
(PDF) Chloride determination in plant tissue using a solid state Chloride Ion Determination A simple, direct and accurate method for chloride determination. Beside acid/base titrations, the titrimetric determination of chloride is one of the most frequently used titrimetric methods of analysis. Theory of determination of chloride in water. The concentration of chloride ions is determined by subtracting the titration findings of the moles of silver ions that reacted with the thiocyanate. The agno. Chloride Ion Determination.
From www.youtube.com
Automated Determination of Chloride in Concrete YouTube Chloride Ion Determination The agno 3 reacts with chloride ion in a 1:1 ratio. A simple, direct and accurate method for chloride determination. The concentration of chloride ions is determined by subtracting the titration findings of the moles of silver ions that reacted with the thiocyanate. The amount of chloride in water can be simply determined by titrating the collected water sample with. Chloride Ion Determination.
From www.researchgate.net
Determination of chloride ions in the presence of some common Chloride Ion Determination Theory of determination of chloride in water. A simple, direct and accurate method for chloride determination. In this experiment, the amount of chloride in an unknown sample was determined. The agno 3 reacts with chloride ion in a 1:1 ratio. This procedure will determine the concentration of chloride ion with a chloride specific ion electrode using potentiometry. Beside acid/base titrations,. Chloride Ion Determination.
From studylib.net
Determination of Chloride Ions in Water Chloride Ion Determination The agno 3 reacts with chloride ion in a 1:1 ratio. The amount of chloride in water can be simply determined by titrating the collected water sample with silver nitrate solution by using potassium chromate indicator. This procedure will determine the concentration of chloride ion with a chloride specific ion electrode using potentiometry. A simple, direct and accurate method for. Chloride Ion Determination.
From es.slideshare.net
Determination of chloride content in water by Volhard method Chloride Ion Determination The agno 3 reacts with chloride ion in a 1:1 ratio. The concentration of chloride ions is determined by subtracting the titration findings of the moles of silver ions that reacted with the thiocyanate. The amount of chloride in water can be simply determined by titrating the collected water sample with silver nitrate solution by using potassium chromate indicator. Beside. Chloride Ion Determination.
From depositphotos.com
Test Chloride Infographic Diagram Showing Laboratory Experiment Chloride Ion Determination Beside acid/base titrations, the titrimetric determination of chloride is one of the most frequently used titrimetric methods of analysis. In this experiment, the amount of chloride in an unknown sample was determined. This procedure will determine the concentration of chloride ion with a chloride specific ion electrode using potentiometry. The agno 3 reacts with chloride ion in a 1:1 ratio.. Chloride Ion Determination.
From www.chegg.com
Solved Experiment 8 Determination of chloride ion in water Chloride Ion Determination A simple, direct and accurate method for chloride determination. The amount of chloride in water can be simply determined by titrating the collected water sample with silver nitrate solution by using potassium chromate indicator. The concentration of chloride ions is determined by subtracting the titration findings of the moles of silver ions that reacted with the thiocyanate. In this experiment,. Chloride Ion Determination.