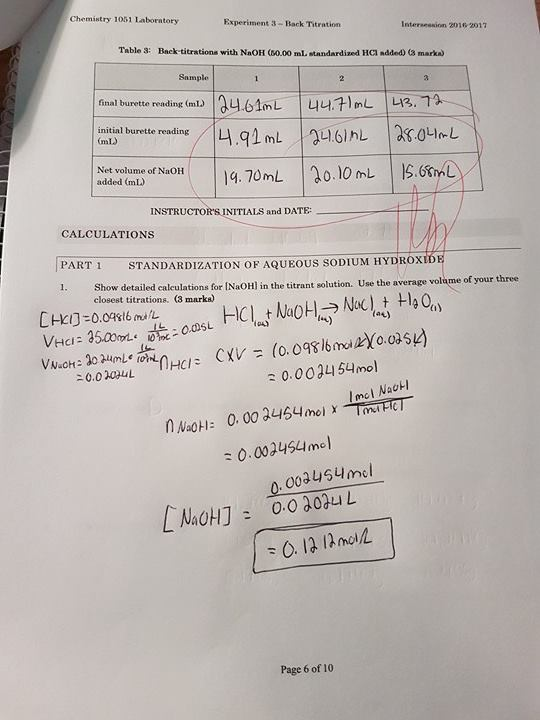
Lab Report On Back Titration . Back titration is used to find the number of moles of a substance by reacting it with an excess volume of reactant of known concentration. Be able to determine the molar mass of a solid monoprotic acid from titration data. Reactant a of unknown concentration is reacted with excess. The resulting mixture is then titrated to work out the number of moles of the reactant in excess. Be able to determine the k a or k b from ph data associated with the titration of a weak acid or base. Back titration is different from the direct. Titration is the process of determining the concentration of an unknown solution using a solution of known concentration. A technique called back titration is used to determine how much stomach acid is neutralized. You will use the naoh you. Be able to calculate k a1 and k a2 for a polyprotic acid. A titration is an analytical procedure used to determine the accurate concentration of a sample by reacting it with a standard solution. This experiment is designed to illustrate techniques used in a typical indirect or back titration.
from www.chegg.com
A technique called back titration is used to determine how much stomach acid is neutralized. Be able to calculate k a1 and k a2 for a polyprotic acid. Back titration is used to find the number of moles of a substance by reacting it with an excess volume of reactant of known concentration. Back titration is different from the direct. Be able to determine the k a or k b from ph data associated with the titration of a weak acid or base. Titration is the process of determining the concentration of an unknown solution using a solution of known concentration. This experiment is designed to illustrate techniques used in a typical indirect or back titration. You will use the naoh you. A titration is an analytical procedure used to determine the accurate concentration of a sample by reacting it with a standard solution. Reactant a of unknown concentration is reacted with excess.
Solved Chemistry 1051 Laboratory Experiment 3 Back
Lab Report On Back Titration A technique called back titration is used to determine how much stomach acid is neutralized. You will use the naoh you. Be able to determine the molar mass of a solid monoprotic acid from titration data. Reactant a of unknown concentration is reacted with excess. Back titration is used to find the number of moles of a substance by reacting it with an excess volume of reactant of known concentration. Back titration is different from the direct. Titration is the process of determining the concentration of an unknown solution using a solution of known concentration. Be able to calculate k a1 and k a2 for a polyprotic acid. Be able to determine the k a or k b from ph data associated with the titration of a weak acid or base. The resulting mixture is then titrated to work out the number of moles of the reactant in excess. This experiment is designed to illustrate techniques used in a typical indirect or back titration. A titration is an analytical procedure used to determine the accurate concentration of a sample by reacting it with a standard solution. A technique called back titration is used to determine how much stomach acid is neutralized.
From www.slideshare.net
Titration report Lab Report On Back Titration This experiment is designed to illustrate techniques used in a typical indirect or back titration. Back titration is different from the direct. Be able to determine the molar mass of a solid monoprotic acid from titration data. Be able to determine the k a or k b from ph data associated with the titration of a weak acid or base.. Lab Report On Back Titration.
From mavink.com
Acid Base Titration Lab Procedure Lab Report On Back Titration The resulting mixture is then titrated to work out the number of moles of the reactant in excess. Reactant a of unknown concentration is reacted with excess. This experiment is designed to illustrate techniques used in a typical indirect or back titration. Be able to determine the molar mass of a solid monoprotic acid from titration data. You will use. Lab Report On Back Titration.
From haileeqohicks.blogspot.com
Acid Base Titration Experiment Report HaileeqoHicks Lab Report On Back Titration Be able to determine the molar mass of a solid monoprotic acid from titration data. Titration is the process of determining the concentration of an unknown solution using a solution of known concentration. Reactant a of unknown concentration is reacted with excess. The resulting mixture is then titrated to work out the number of moles of the reactant in excess.. Lab Report On Back Titration.
From www.scribd.com
chemistry lab report (back titration) Titration Acid Lab Report On Back Titration The resulting mixture is then titrated to work out the number of moles of the reactant in excess. Back titration is different from the direct. A technique called back titration is used to determine how much stomach acid is neutralized. Be able to determine the molar mass of a solid monoprotic acid from titration data. Be able to calculate k. Lab Report On Back Titration.
From www.chegg.com
Solved Chemistry 1051 Laboratory Experiment 3 Back Lab Report On Back Titration A titration is an analytical procedure used to determine the accurate concentration of a sample by reacting it with a standard solution. Back titration is different from the direct. Be able to determine the k a or k b from ph data associated with the titration of a weak acid or base. A technique called back titration is used to. Lab Report On Back Titration.
From www.scribd.com
Titration Lab Report PDF Titration Chemistry Lab Report On Back Titration Be able to calculate k a1 and k a2 for a polyprotic acid. The resulting mixture is then titrated to work out the number of moles of the reactant in excess. This experiment is designed to illustrate techniques used in a typical indirect or back titration. Back titration is used to find the number of moles of a substance by. Lab Report On Back Titration.
From www.studypool.com
SOLUTION CHM 113 Experiment 8 Acid Base Titration Lab Report Studypool Lab Report On Back Titration Be able to determine the molar mass of a solid monoprotic acid from titration data. A titration is an analytical procedure used to determine the accurate concentration of a sample by reacting it with a standard solution. Back titration is different from the direct. Reactant a of unknown concentration is reacted with excess. You will use the naoh you. This. Lab Report On Back Titration.
From nerdyseal.com
Back titration lab report 655 Words NerdySeal Lab Report On Back Titration Reactant a of unknown concentration is reacted with excess. Titration is the process of determining the concentration of an unknown solution using a solution of known concentration. Be able to calculate k a1 and k a2 for a polyprotic acid. Be able to determine the k a or k b from ph data associated with the titration of a weak. Lab Report On Back Titration.
From www.studocu.com
Titration Lab Report Titration Lab Report Objective To find the Lab Report On Back Titration Back titration is used to find the number of moles of a substance by reacting it with an excess volume of reactant of known concentration. Titration is the process of determining the concentration of an unknown solution using a solution of known concentration. A technique called back titration is used to determine how much stomach acid is neutralized. Back titration. Lab Report On Back Titration.
From bazaemmalyman.blogspot.com
Back Titration Method Emma Lyman Lab Report On Back Titration Reactant a of unknown concentration is reacted with excess. The resulting mixture is then titrated to work out the number of moles of the reactant in excess. Be able to determine the k a or k b from ph data associated with the titration of a weak acid or base. This experiment is designed to illustrate techniques used in a. Lab Report On Back Titration.
From www.youtube.com
Back Titration Calculations // HSC Chemistry YouTube Lab Report On Back Titration Back titration is used to find the number of moles of a substance by reacting it with an excess volume of reactant of known concentration. Be able to calculate k a1 and k a2 for a polyprotic acid. A titration is an analytical procedure used to determine the accurate concentration of a sample by reacting it with a standard solution.. Lab Report On Back Titration.
From www.studypool.com
SOLUTION Chem lab report objective to determine the content of vitamin Lab Report On Back Titration Reactant a of unknown concentration is reacted with excess. A titration is an analytical procedure used to determine the accurate concentration of a sample by reacting it with a standard solution. You will use the naoh you. This experiment is designed to illustrate techniques used in a typical indirect or back titration. Back titration is used to find the number. Lab Report On Back Titration.
From www.coursehero.com
[Solved] Analytical chemistry ACIDBASED TITRATION II (BACK TITRATION Lab Report On Back Titration Titration is the process of determining the concentration of an unknown solution using a solution of known concentration. This experiment is designed to illustrate techniques used in a typical indirect or back titration. You will use the naoh you. Back titration is different from the direct. Be able to calculate k a1 and k a2 for a polyprotic acid. The. Lab Report On Back Titration.
From www.studocu.com
BackTitration F22 lab BackTitration of a Commercial Antacid Lab Report On Back Titration Back titration is used to find the number of moles of a substance by reacting it with an excess volume of reactant of known concentration. This experiment is designed to illustrate techniques used in a typical indirect or back titration. Be able to determine the k a or k b from ph data associated with the titration of a weak. Lab Report On Back Titration.
From chemistry.stackexchange.com
stoichiometry Novalucol back titration lab calculations Chemistry Lab Report On Back Titration A technique called back titration is used to determine how much stomach acid is neutralized. Back titration is different from the direct. Reactant a of unknown concentration is reacted with excess. This experiment is designed to illustrate techniques used in a typical indirect or back titration. Back titration is used to find the number of moles of a substance by. Lab Report On Back Titration.
From www.pdfprof.com
hydrolysis of aspirin lab report Lab Report On Back Titration Be able to determine the k a or k b from ph data associated with the titration of a weak acid or base. Back titration is different from the direct. This experiment is designed to illustrate techniques used in a typical indirect or back titration. Be able to calculate k a1 and k a2 for a polyprotic acid. Be able. Lab Report On Back Titration.
From studylib.net
CHEMISTRY I LAB INTRO TO TITRATION Lab Report On Back Titration Be able to determine the molar mass of a solid monoprotic acid from titration data. This experiment is designed to illustrate techniques used in a typical indirect or back titration. Titration is the process of determining the concentration of an unknown solution using a solution of known concentration. The resulting mixture is then titrated to work out the number of. Lab Report On Back Titration.
From mavink.com
Acid Base Titration Lab Procedure Lab Report On Back Titration Be able to calculate k a1 and k a2 for a polyprotic acid. Reactant a of unknown concentration is reacted with excess. A titration is an analytical procedure used to determine the accurate concentration of a sample by reacting it with a standard solution. Be able to determine the k a or k b from ph data associated with the. Lab Report On Back Titration.
From www.studocu.com
CHEM 110L Experiment 10 It's Back... Titration v2 Experiment 10 It Lab Report On Back Titration This experiment is designed to illustrate techniques used in a typical indirect or back titration. Reactant a of unknown concentration is reacted with excess. You will use the naoh you. Be able to determine the molar mass of a solid monoprotic acid from titration data. Back titration is used to find the number of moles of a substance by reacting. Lab Report On Back Titration.
From www.scribd.com
LAB Report 3 Titration Chemistry Lab Report On Back Titration Back titration is different from the direct. This experiment is designed to illustrate techniques used in a typical indirect or back titration. You will use the naoh you. Reactant a of unknown concentration is reacted with excess. The resulting mixture is then titrated to work out the number of moles of the reactant in excess. A titration is an analytical. Lab Report On Back Titration.
From www.chegg.com
Solved Antacid Analysis and TitrationLab Report Assistant Lab Report On Back Titration Reactant a of unknown concentration is reacted with excess. Titration is the process of determining the concentration of an unknown solution using a solution of known concentration. A titration is an analytical procedure used to determine the accurate concentration of a sample by reacting it with a standard solution. Be able to determine the k a or k b from. Lab Report On Back Titration.
From www.studocu.com
Lab 7 p H and titration lab report Name Lab Report On Back Titration The resulting mixture is then titrated to work out the number of moles of the reactant in excess. Be able to determine the k a or k b from ph data associated with the titration of a weak acid or base. Be able to determine the molar mass of a solid monoprotic acid from titration data. This experiment is designed. Lab Report On Back Titration.
From www.studocu.com
Back Titration Lab Report Lab Report 4/11/ Determination of Na2CO3 in Lab Report On Back Titration Be able to determine the k a or k b from ph data associated with the titration of a weak acid or base. A titration is an analytical procedure used to determine the accurate concentration of a sample by reacting it with a standard solution. Back titration is different from the direct. Titration is the process of determining the concentration. Lab Report On Back Titration.
From www.scribd.com
titration lab report Titration Ph Lab Report On Back Titration Be able to determine the k a or k b from ph data associated with the titration of a weak acid or base. This experiment is designed to illustrate techniques used in a typical indirect or back titration. Back titration is used to find the number of moles of a substance by reacting it with an excess volume of reactant. Lab Report On Back Titration.
From studylib.net
Titration Lab Report Walker Singleton Lab Report On Back Titration You will use the naoh you. Be able to determine the molar mass of a solid monoprotic acid from titration data. Be able to calculate k a1 and k a2 for a polyprotic acid. Reactant a of unknown concentration is reacted with excess. Back titration is different from the direct. Back titration is used to find the number of moles. Lab Report On Back Titration.
From www.studocu.com
Titration Lab Report About the lab tiration Titration Lab Report Lab Report On Back Titration You will use the naoh you. Be able to determine the molar mass of a solid monoprotic acid from titration data. Back titration is different from the direct. Titration is the process of determining the concentration of an unknown solution using a solution of known concentration. Back titration is used to find the number of moles of a substance by. Lab Report On Back Titration.
From www.scribd.com
Analysis of An Antacid Using Back Titration (CAPE LAB) PDF Lab Report On Back Titration Back titration is used to find the number of moles of a substance by reacting it with an excess volume of reactant of known concentration. Titration is the process of determining the concentration of an unknown solution using a solution of known concentration. This experiment is designed to illustrate techniques used in a typical indirect or back titration. A titration. Lab Report On Back Titration.
From www.youtube.com
Lab Back titration to determine the CaCO3 in eggshell DATA Lab Report On Back Titration The resulting mixture is then titrated to work out the number of moles of the reactant in excess. Be able to determine the k a or k b from ph data associated with the titration of a weak acid or base. A technique called back titration is used to determine how much stomach acid is neutralized. Titration is the process. Lab Report On Back Titration.
From vasadylanpeake.blogspot.com
Back Titration Method Dylan Peake Lab Report On Back Titration A titration is an analytical procedure used to determine the accurate concentration of a sample by reacting it with a standard solution. Be able to determine the molar mass of a solid monoprotic acid from titration data. Be able to determine the k a or k b from ph data associated with the titration of a weak acid or base.. Lab Report On Back Titration.
From www.scribd.com
Lab Report Sample[1] Titration Chemistry Lab Report On Back Titration Titration is the process of determining the concentration of an unknown solution using a solution of known concentration. Be able to determine the k a or k b from ph data associated with the titration of a weak acid or base. Be able to determine the molar mass of a solid monoprotic acid from titration data. Back titration is different. Lab Report On Back Titration.
From www.studocu.com
Back Titrationlab4 lab report Determination of Aspirin using Back Lab Report On Back Titration A technique called back titration is used to determine how much stomach acid is neutralized. This experiment is designed to illustrate techniques used in a typical indirect or back titration. Be able to determine the k a or k b from ph data associated with the titration of a weak acid or base. Be able to determine the molar mass. Lab Report On Back Titration.
From www.chemicals.co.uk
How To Carry Out a Titration Experiment The Chemistry Blog Lab Report On Back Titration Be able to calculate k a1 and k a2 for a polyprotic acid. A technique called back titration is used to determine how much stomach acid is neutralized. Titration is the process of determining the concentration of an unknown solution using a solution of known concentration. Back titration is different from the direct. Reactant a of unknown concentration is reacted. Lab Report On Back Titration.
From www.scribd.com
chemistry lab report titration lab Molar Concentration Mole (Unit) Lab Report On Back Titration Titration is the process of determining the concentration of an unknown solution using a solution of known concentration. Be able to calculate k a1 and k a2 for a polyprotic acid. The resulting mixture is then titrated to work out the number of moles of the reactant in excess. You will use the naoh you. Back titration is different from. Lab Report On Back Titration.
From studylib.net
Determination of aspirin in tablets using back titration Lab Report On Back Titration A titration is an analytical procedure used to determine the accurate concentration of a sample by reacting it with a standard solution. Back titration is different from the direct. You will use the naoh you. Be able to determine the molar mass of a solid monoprotic acid from titration data. The resulting mixture is then titrated to work out the. Lab Report On Back Titration.
From www.studypool.com
SOLUTION Conductometric titration II lab report Studypool Lab Report On Back Titration Be able to determine the k a or k b from ph data associated with the titration of a weak acid or base. A technique called back titration is used to determine how much stomach acid is neutralized. Be able to determine the molar mass of a solid monoprotic acid from titration data. Be able to calculate k a1 and. Lab Report On Back Titration.