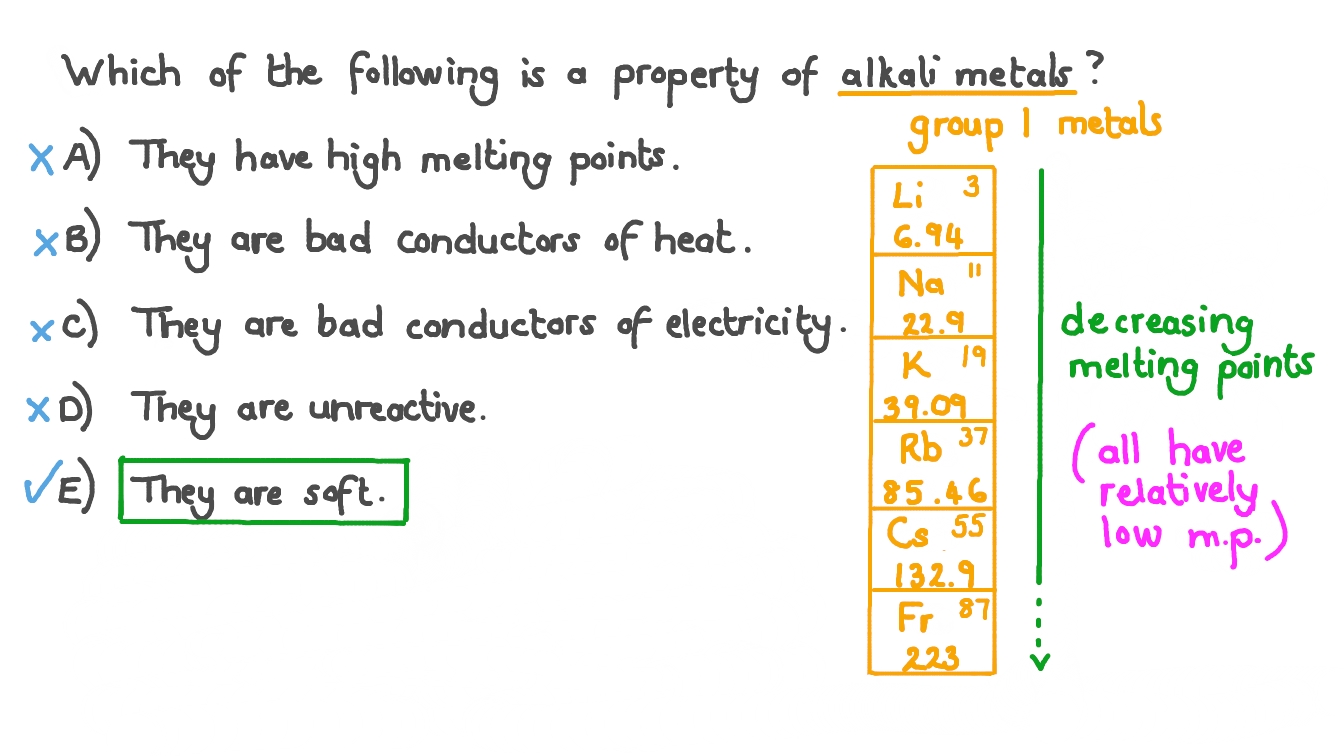
What Are The Common Physical Properties Of Alkali Metals . The group 1 elements are all soft, reactive metals with low melting points. The table summarizes the important physical and thermodynamic properties of the alkali metals. Many of the physical properties of the alkali metals (table \(\pageindex{3}\).4) are typical of metals, e.g.,. Alkali metals have one electron in their outer shell, which is loosely bound. The alkali metals tend to form +1 cations. The lone outer shell electrons leads the alkali metal elements to share several common properties: The one outer electron is easily lost, forming the univalent (1+). They react with water to produce an alkaline metal hydroxide solution. Cation formation is favored by the relatively low ionization energies of the free metal (which makes it easier to form the cation) and the high. The alkali metals exhibit many of the physical properties common to metals, although their densities are lower than those of other metals.
from www.nagwa.com
Many of the physical properties of the alkali metals (table \(\pageindex{3}\).4) are typical of metals, e.g.,. They react with water to produce an alkaline metal hydroxide solution. Alkali metals have one electron in their outer shell, which is loosely bound. Cation formation is favored by the relatively low ionization energies of the free metal (which makes it easier to form the cation) and the high. The one outer electron is easily lost, forming the univalent (1+). The alkali metals exhibit many of the physical properties common to metals, although their densities are lower than those of other metals. The lone outer shell electrons leads the alkali metal elements to share several common properties: The table summarizes the important physical and thermodynamic properties of the alkali metals. The group 1 elements are all soft, reactive metals with low melting points. The alkali metals tend to form +1 cations.
Question Video Identifying the Property of Alkali Metals From a List
What Are The Common Physical Properties Of Alkali Metals They react with water to produce an alkaline metal hydroxide solution. Alkali metals have one electron in their outer shell, which is loosely bound. Cation formation is favored by the relatively low ionization energies of the free metal (which makes it easier to form the cation) and the high. The one outer electron is easily lost, forming the univalent (1+). They react with water to produce an alkaline metal hydroxide solution. The alkali metals tend to form +1 cations. The alkali metals exhibit many of the physical properties common to metals, although their densities are lower than those of other metals. The lone outer shell electrons leads the alkali metal elements to share several common properties: The table summarizes the important physical and thermodynamic properties of the alkali metals. Many of the physical properties of the alkali metals (table \(\pageindex{3}\).4) are typical of metals, e.g.,. The group 1 elements are all soft, reactive metals with low melting points.
From www.slideserve.com
PPT Elements and Their Properties PowerPoint Presentation, free What Are The Common Physical Properties Of Alkali Metals The lone outer shell electrons leads the alkali metal elements to share several common properties: Alkali metals have one electron in their outer shell, which is loosely bound. Many of the physical properties of the alkali metals (table \(\pageindex{3}\).4) are typical of metals, e.g.,. Cation formation is favored by the relatively low ionization energies of the free metal (which makes. What Are The Common Physical Properties Of Alkali Metals.
From schoolworkhelper.net
Metals Chemical & Physical properties SchoolWorkHelper What Are The Common Physical Properties Of Alkali Metals Alkali metals have one electron in their outer shell, which is loosely bound. Many of the physical properties of the alkali metals (table \(\pageindex{3}\).4) are typical of metals, e.g.,. The one outer electron is easily lost, forming the univalent (1+). The table summarizes the important physical and thermodynamic properties of the alkali metals. The alkali metals tend to form +1. What Are The Common Physical Properties Of Alkali Metals.
From www.sliderbase.com
Element Classes Presentation Chemistry What Are The Common Physical Properties Of Alkali Metals The alkali metals tend to form +1 cations. The table summarizes the important physical and thermodynamic properties of the alkali metals. The group 1 elements are all soft, reactive metals with low melting points. Cation formation is favored by the relatively low ionization energies of the free metal (which makes it easier to form the cation) and the high. Alkali. What Are The Common Physical Properties Of Alkali Metals.
From www.slideserve.com
PPT KS3 Chemistry PowerPoint Presentation, free download ID3804641 What Are The Common Physical Properties Of Alkali Metals The alkali metals tend to form +1 cations. Many of the physical properties of the alkali metals (table \(\pageindex{3}\).4) are typical of metals, e.g.,. The table summarizes the important physical and thermodynamic properties of the alkali metals. Cation formation is favored by the relatively low ionization energies of the free metal (which makes it easier to form the cation) and. What Are The Common Physical Properties Of Alkali Metals.
From www.slideserve.com
PPT Physical Properties of Metals PowerPoint Presentation ID5519685 What Are The Common Physical Properties Of Alkali Metals The group 1 elements are all soft, reactive metals with low melting points. Alkali metals have one electron in their outer shell, which is loosely bound. The one outer electron is easily lost, forming the univalent (1+). Cation formation is favored by the relatively low ionization energies of the free metal (which makes it easier to form the cation) and. What Are The Common Physical Properties Of Alkali Metals.
From joifkoaln.blob.core.windows.net
What Properties Do Alkali Metals Have at Christopher Vinson blog What Are The Common Physical Properties Of Alkali Metals The one outer electron is easily lost, forming the univalent (1+). Alkali metals have one electron in their outer shell, which is loosely bound. The table summarizes the important physical and thermodynamic properties of the alkali metals. Many of the physical properties of the alkali metals (table \(\pageindex{3}\).4) are typical of metals, e.g.,. The alkali metals tend to form +1. What Are The Common Physical Properties Of Alkali Metals.
From assign.unaux.com
Periodic properties of alkali metals and halogens. assign What Are The Common Physical Properties Of Alkali Metals The lone outer shell electrons leads the alkali metal elements to share several common properties: The alkali metals tend to form +1 cations. Cation formation is favored by the relatively low ionization energies of the free metal (which makes it easier to form the cation) and the high. The one outer electron is easily lost, forming the univalent (1+). Many. What Are The Common Physical Properties Of Alkali Metals.
From www.slideserve.com
PPT Alkaline Earth Metals PowerPoint Presentation ID2046855 What Are The Common Physical Properties Of Alkali Metals Cation formation is favored by the relatively low ionization energies of the free metal (which makes it easier to form the cation) and the high. The alkali metals tend to form +1 cations. The table summarizes the important physical and thermodynamic properties of the alkali metals. The lone outer shell electrons leads the alkali metal elements to share several common. What Are The Common Physical Properties Of Alkali Metals.
From spmchemistry.blog.onlinetuition.com.my
Physical Properties of Alkali Metals SPM Chemistry What Are The Common Physical Properties Of Alkali Metals The lone outer shell electrons leads the alkali metal elements to share several common properties: The alkali metals tend to form +1 cations. Alkali metals have one electron in their outer shell, which is loosely bound. The alkali metals exhibit many of the physical properties common to metals, although their densities are lower than those of other metals. Cation formation. What Are The Common Physical Properties Of Alkali Metals.
From chemistrysources.com
alkali metals (group 1 elements) مصادر الكيمياء What Are The Common Physical Properties Of Alkali Metals They react with water to produce an alkaline metal hydroxide solution. The table summarizes the important physical and thermodynamic properties of the alkali metals. The alkali metals tend to form +1 cations. The one outer electron is easily lost, forming the univalent (1+). The alkali metals exhibit many of the physical properties common to metals, although their densities are lower. What Are The Common Physical Properties Of Alkali Metals.
From www.slideserve.com
PPT Elements and Their Properties PowerPoint Presentation, free What Are The Common Physical Properties Of Alkali Metals They react with water to produce an alkaline metal hydroxide solution. The one outer electron is easily lost, forming the univalent (1+). The alkali metals exhibit many of the physical properties common to metals, although their densities are lower than those of other metals. The table summarizes the important physical and thermodynamic properties of the alkali metals. The group 1. What Are The Common Physical Properties Of Alkali Metals.
From exobgridh.blob.core.windows.net
What Are The Common Physical Properties Of Alkali Metals at Israel What Are The Common Physical Properties Of Alkali Metals Many of the physical properties of the alkali metals (table \(\pageindex{3}\).4) are typical of metals, e.g.,. The group 1 elements are all soft, reactive metals with low melting points. They react with water to produce an alkaline metal hydroxide solution. Cation formation is favored by the relatively low ionization energies of the free metal (which makes it easier to form. What Are The Common Physical Properties Of Alkali Metals.
From overallscience.com
Trends in atomic and physical properties of alkali metals Overall Science What Are The Common Physical Properties Of Alkali Metals They react with water to produce an alkaline metal hydroxide solution. The one outer electron is easily lost, forming the univalent (1+). Many of the physical properties of the alkali metals (table \(\pageindex{3}\).4) are typical of metals, e.g.,. Alkali metals have one electron in their outer shell, which is loosely bound. Cation formation is favored by the relatively low ionization. What Are The Common Physical Properties Of Alkali Metals.
From www.vedantu.com
Alkali Metals Chemical Elements, Properties Alkali Metals Periodic What Are The Common Physical Properties Of Alkali Metals Many of the physical properties of the alkali metals (table \(\pageindex{3}\).4) are typical of metals, e.g.,. The table summarizes the important physical and thermodynamic properties of the alkali metals. Alkali metals have one electron in their outer shell, which is loosely bound. The one outer electron is easily lost, forming the univalent (1+). The alkali metals exhibit many of the. What Are The Common Physical Properties Of Alkali Metals.
From pediaa.com
Difference Between Alkali Metals and Alkaline Earth Metals Definition What Are The Common Physical Properties Of Alkali Metals The lone outer shell electrons leads the alkali metal elements to share several common properties: They react with water to produce an alkaline metal hydroxide solution. The group 1 elements are all soft, reactive metals with low melting points. The table summarizes the important physical and thermodynamic properties of the alkali metals. The one outer electron is easily lost, forming. What Are The Common Physical Properties Of Alkali Metals.
From www.slideshare.net
Physical and chemical properties of alkali metals What Are The Common Physical Properties Of Alkali Metals They react with water to produce an alkaline metal hydroxide solution. The one outer electron is easily lost, forming the univalent (1+). Cation formation is favored by the relatively low ionization energies of the free metal (which makes it easier to form the cation) and the high. The group 1 elements are all soft, reactive metals with low melting points.. What Are The Common Physical Properties Of Alkali Metals.
From www.studypool.com
SOLUTION Physical chemical properties of alkali and alkaline earth What Are The Common Physical Properties Of Alkali Metals The lone outer shell electrons leads the alkali metal elements to share several common properties: The alkali metals exhibit many of the physical properties common to metals, although their densities are lower than those of other metals. The one outer electron is easily lost, forming the univalent (1+). The alkali metals tend to form +1 cations. The group 1 elements. What Are The Common Physical Properties Of Alkali Metals.
From xlskoor.blogspot.com
Alkali Metals Chemistry What Are The Common Physical Properties Of Alkali Metals The one outer electron is easily lost, forming the univalent (1+). They react with water to produce an alkaline metal hydroxide solution. The table summarizes the important physical and thermodynamic properties of the alkali metals. The group 1 elements are all soft, reactive metals with low melting points. Cation formation is favored by the relatively low ionization energies of the. What Are The Common Physical Properties Of Alkali Metals.
From www.youtube.com
Physical properties of alkali metals YouTube What Are The Common Physical Properties Of Alkali Metals The group 1 elements are all soft, reactive metals with low melting points. The alkali metals exhibit many of the physical properties common to metals, although their densities are lower than those of other metals. The table summarizes the important physical and thermodynamic properties of the alkali metals. The alkali metals tend to form +1 cations. Cation formation is favored. What Are The Common Physical Properties Of Alkali Metals.
From www.nagwa.com
Question Video Identifying the Property of Alkali Metals From a List What Are The Common Physical Properties Of Alkali Metals The one outer electron is easily lost, forming the univalent (1+). The alkali metals tend to form +1 cations. The alkali metals exhibit many of the physical properties common to metals, although their densities are lower than those of other metals. Alkali metals have one electron in their outer shell, which is loosely bound. They react with water to produce. What Are The Common Physical Properties Of Alkali Metals.
From studylib.net
Alkali metals What Are The Common Physical Properties Of Alkali Metals The one outer electron is easily lost, forming the univalent (1+). They react with water to produce an alkaline metal hydroxide solution. Cation formation is favored by the relatively low ionization energies of the free metal (which makes it easier to form the cation) and the high. The alkali metals exhibit many of the physical properties common to metals, although. What Are The Common Physical Properties Of Alkali Metals.
From www.expii.com
Alkali Metals — Overview & Properties Expii What Are The Common Physical Properties Of Alkali Metals The one outer electron is easily lost, forming the univalent (1+). Many of the physical properties of the alkali metals (table \(\pageindex{3}\).4) are typical of metals, e.g.,. The group 1 elements are all soft, reactive metals with low melting points. The table summarizes the important physical and thermodynamic properties of the alkali metals. The alkali metals exhibit many of the. What Are The Common Physical Properties Of Alkali Metals.
From www.britannica.com
alkali metal Definition, Properties, & Facts Britannica What Are The Common Physical Properties Of Alkali Metals Many of the physical properties of the alkali metals (table \(\pageindex{3}\).4) are typical of metals, e.g.,. The lone outer shell electrons leads the alkali metal elements to share several common properties: The one outer electron is easily lost, forming the univalent (1+). Cation formation is favored by the relatively low ionization energies of the free metal (which makes it easier. What Are The Common Physical Properties Of Alkali Metals.
From www.slideshare.net
The Periodic Table What Are The Common Physical Properties Of Alkali Metals The group 1 elements are all soft, reactive metals with low melting points. Many of the physical properties of the alkali metals (table \(\pageindex{3}\).4) are typical of metals, e.g.,. The lone outer shell electrons leads the alkali metal elements to share several common properties: Cation formation is favored by the relatively low ionization energies of the free metal (which makes. What Are The Common Physical Properties Of Alkali Metals.
From www.indcareer.com
Physical Properties of Alkali Metals IndCareer Schools What Are The Common Physical Properties Of Alkali Metals The group 1 elements are all soft, reactive metals with low melting points. Alkali metals have one electron in their outer shell, which is loosely bound. Many of the physical properties of the alkali metals (table \(\pageindex{3}\).4) are typical of metals, e.g.,. They react with water to produce an alkaline metal hydroxide solution. The one outer electron is easily lost,. What Are The Common Physical Properties Of Alkali Metals.
From www.slideserve.com
PPT ELEMENT CLASSES PowerPoint Presentation ID149914 What Are The Common Physical Properties Of Alkali Metals The lone outer shell electrons leads the alkali metal elements to share several common properties: Cation formation is favored by the relatively low ionization energies of the free metal (which makes it easier to form the cation) and the high. The group 1 elements are all soft, reactive metals with low melting points. The table summarizes the important physical and. What Are The Common Physical Properties Of Alkali Metals.
From www.slideshare.net
Metals Physical Properties What Are The Common Physical Properties Of Alkali Metals Alkali metals have one electron in their outer shell, which is loosely bound. The alkali metals exhibit many of the physical properties common to metals, although their densities are lower than those of other metals. The lone outer shell electrons leads the alkali metal elements to share several common properties: Cation formation is favored by the relatively low ionization energies. What Are The Common Physical Properties Of Alkali Metals.
From elchoroukhost.net
Properties Of Alkali Metals On The Periodic Table Elcho Table What Are The Common Physical Properties Of Alkali Metals The alkali metals exhibit many of the physical properties common to metals, although their densities are lower than those of other metals. The alkali metals tend to form +1 cations. The lone outer shell electrons leads the alkali metal elements to share several common properties: They react with water to produce an alkaline metal hydroxide solution. Many of the physical. What Are The Common Physical Properties Of Alkali Metals.
From exobgridh.blob.core.windows.net
What Are The Common Physical Properties Of Alkali Metals at Israel What Are The Common Physical Properties Of Alkali Metals The one outer electron is easily lost, forming the univalent (1+). They react with water to produce an alkaline metal hydroxide solution. The alkali metals exhibit many of the physical properties common to metals, although their densities are lower than those of other metals. The table summarizes the important physical and thermodynamic properties of the alkali metals. The lone outer. What Are The Common Physical Properties Of Alkali Metals.
From www.slideserve.com
PPT Group 1 The alkali metals PowerPoint Presentation ID5525387 What Are The Common Physical Properties Of Alkali Metals The one outer electron is easily lost, forming the univalent (1+). The group 1 elements are all soft, reactive metals with low melting points. The lone outer shell electrons leads the alkali metal elements to share several common properties: The alkali metals tend to form +1 cations. The table summarizes the important physical and thermodynamic properties of the alkali metals.. What Are The Common Physical Properties Of Alkali Metals.
From spmchemistry.blog.onlinetuition.com.my
Physical Properties of Alkali Metals SPM Chemistry What Are The Common Physical Properties Of Alkali Metals Cation formation is favored by the relatively low ionization energies of the free metal (which makes it easier to form the cation) and the high. Alkali metals have one electron in their outer shell, which is loosely bound. The alkali metals tend to form +1 cations. Many of the physical properties of the alkali metals (table \(\pageindex{3}\).4) are typical of. What Are The Common Physical Properties Of Alkali Metals.
From slideplayer.com
Alkali Metals Electrostructure and reactivity Physical properties ppt What Are The Common Physical Properties Of Alkali Metals The alkali metals tend to form +1 cations. The one outer electron is easily lost, forming the univalent (1+). Alkali metals have one electron in their outer shell, which is loosely bound. The alkali metals exhibit many of the physical properties common to metals, although their densities are lower than those of other metals. The group 1 elements are all. What Are The Common Physical Properties Of Alkali Metals.
From online-learning-college.com
Group 1 alkali metals Properties of alkali metals Reactions What Are The Common Physical Properties Of Alkali Metals The group 1 elements are all soft, reactive metals with low melting points. The one outer electron is easily lost, forming the univalent (1+). They react with water to produce an alkaline metal hydroxide solution. The lone outer shell electrons leads the alkali metal elements to share several common properties: Alkali metals have one electron in their outer shell, which. What Are The Common Physical Properties Of Alkali Metals.
From ravennewsrogers.blogspot.com
Describe the Properties of Alkali Metals What Are The Common Physical Properties Of Alkali Metals The alkali metals exhibit many of the physical properties common to metals, although their densities are lower than those of other metals. The alkali metals tend to form +1 cations. Many of the physical properties of the alkali metals (table \(\pageindex{3}\).4) are typical of metals, e.g.,. They react with water to produce an alkaline metal hydroxide solution. The table summarizes. What Are The Common Physical Properties Of Alkali Metals.
From www.tes.com
Lesson Alkali Metals GCSE Edexcel 91 Teaching Resources What Are The Common Physical Properties Of Alkali Metals The lone outer shell electrons leads the alkali metal elements to share several common properties: They react with water to produce an alkaline metal hydroxide solution. Cation formation is favored by the relatively low ionization energies of the free metal (which makes it easier to form the cation) and the high. The alkali metals exhibit many of the physical properties. What Are The Common Physical Properties Of Alkali Metals.