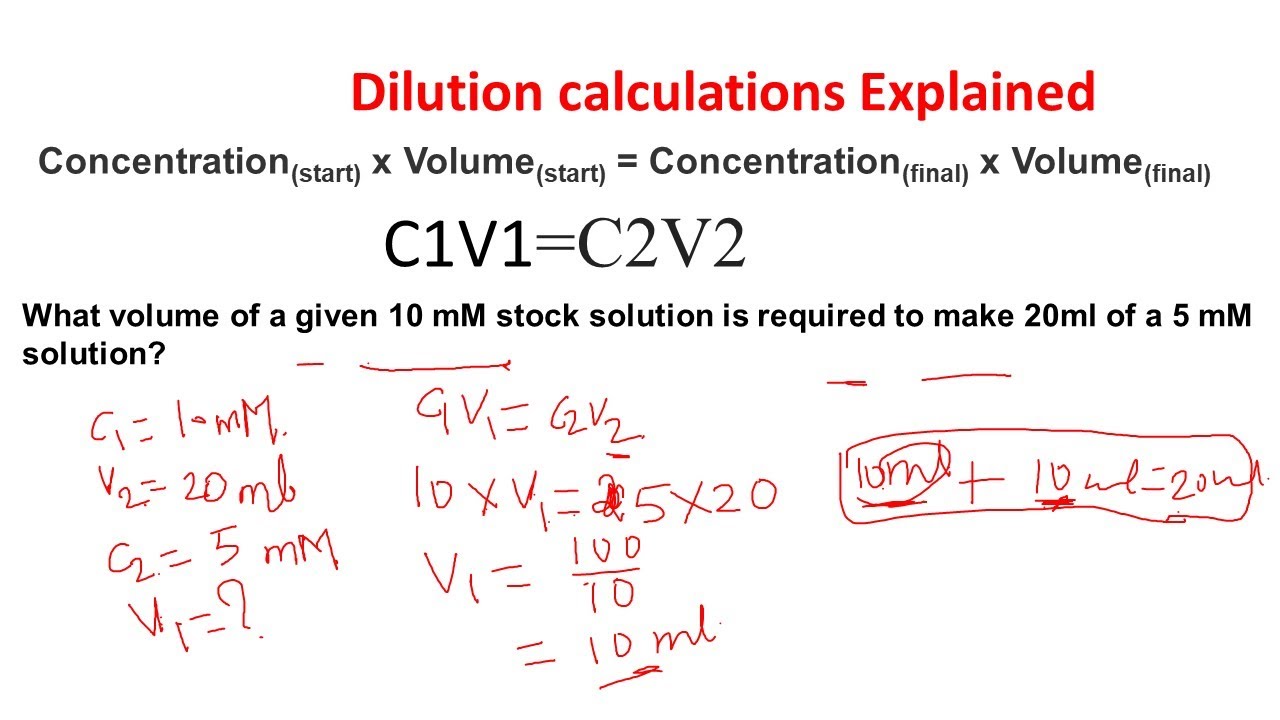
Dilution Equation Ap Chem . 3) use the dilution formula: M 1 v 1 = m 2 v 2 (7.90 m) (133 ml) = (0.648 m) (v 2) v 2 = 1620 ml. the dilution equation is expressed as: this chemistry video tutorial explains how to solve common dilution problems using a simple formula using. this process is known as dilution. We can relate the concentrations and volumes before and after a dilution. This is because the amount of. the solute concentration of a solution may be decreased by adding solvent, a process referred to as dilution. To carry out a dilution, the following equation is. When additional water is added to an aqueous solution, the concentration of that solution decreases. Where c1 and v1 represent the initial concentration and volume of the solution,. dilution is the addition of solvent, which decreases the concentration of the solute in the solution. The dilution equation is a simple. You should dilute the 133 ml of. Concentration is the removal of solvent, which.
from www.youtube.com
M 1 v 1 = m 2 v 2 (7.90 m) (133 ml) = (0.648 m) (v 2) v 2 = 1620 ml. this chemistry video tutorial explains how to solve common dilution problems using a simple formula using. the solute concentration of a solution may be decreased by adding solvent, a process referred to as dilution. To carry out a dilution, the following equation is. dilution is the addition of solvent, which decreases the concentration of the solute in the solution. the dilution equation is expressed as: concentrated stock solution must be diluted to obtain the desired concentration. Where c1 and v1 represent the initial concentration and volume of the solution,. The dilution equation is a simple. We can relate the concentrations and volumes before and after a dilution.
Dilution calculations Dilution problems Stock dilutions Biology and
Dilution Equation Ap Chem 3) use the dilution formula: dilution is the addition of solvent, which decreases the concentration of the solute in the solution. When additional water is added to an aqueous solution, the concentration of that solution decreases. the dilution equation is expressed as: this process is known as dilution. 3) use the dilution formula: the solute concentration of a solution may be decreased by adding solvent, a process referred to as dilution. To carry out a dilution, the following equation is. Concentration is the removal of solvent, which. Where c1 and v1 represent the initial concentration and volume of the solution,. The dilution equation is a simple. concentrated stock solution must be diluted to obtain the desired concentration. This is because the amount of. You should dilute the 133 ml of. M 1 v 1 = m 2 v 2 (7.90 m) (133 ml) = (0.648 m) (v 2) v 2 = 1620 ml. this chemistry video tutorial explains how to solve common dilution problems using a simple formula using.
From www.youtube.com
Chem 11 Dilution Calculations_Solving for Final Volume after Dilution Dilution Equation Ap Chem Where c1 and v1 represent the initial concentration and volume of the solution,. 3) use the dilution formula: the dilution equation is expressed as: Concentration is the removal of solvent, which. This is because the amount of. M 1 v 1 = m 2 v 2 (7.90 m) (133 ml) = (0.648 m) (v 2) v 2 =. Dilution Equation Ap Chem.
From www.youtube.com
CHEMISTRY 101 Solution Dilutions YouTube Dilution Equation Ap Chem The dilution equation is a simple. concentrated stock solution must be diluted to obtain the desired concentration. You should dilute the 133 ml of. We can relate the concentrations and volumes before and after a dilution. M 1 v 1 = m 2 v 2 (7.90 m) (133 ml) = (0.648 m) (v 2) v 2 = 1620 ml.. Dilution Equation Ap Chem.
From www.youtube.com
Dilution calculations Dilution problems Stock dilutions Biology and Dilution Equation Ap Chem M 1 v 1 = m 2 v 2 (7.90 m) (133 ml) = (0.648 m) (v 2) v 2 = 1620 ml. We can relate the concentrations and volumes before and after a dilution. concentrated stock solution must be diluted to obtain the desired concentration. You should dilute the 133 ml of. dilution is the addition of. Dilution Equation Ap Chem.
From www.slideserve.com
PPT Analytical Chemistry PowerPoint Presentation, free download ID Dilution Equation Ap Chem 3) use the dilution formula: Where c1 and v1 represent the initial concentration and volume of the solution,. M 1 v 1 = m 2 v 2 (7.90 m) (133 ml) = (0.648 m) (v 2) v 2 = 1620 ml. The dilution equation is a simple. dilution is the addition of solvent, which decreases the concentration of. Dilution Equation Ap Chem.
From www.scribd.com
Dilution (Equation) Wikipedia PDF Solution Chemistry Dilution Equation Ap Chem 3) use the dilution formula: We can relate the concentrations and volumes before and after a dilution. this process is known as dilution. You should dilute the 133 ml of. This is because the amount of. dilution is the addition of solvent, which decreases the concentration of the solute in the solution. Where c1 and v1 represent. Dilution Equation Ap Chem.
From giobwgqpk.blob.core.windows.net
Dilution Formula Explained at Patti Tejada blog Dilution Equation Ap Chem Concentration is the removal of solvent, which. the dilution equation is expressed as: concentrated stock solution must be diluted to obtain the desired concentration. When additional water is added to an aqueous solution, the concentration of that solution decreases. M 1 v 1 = m 2 v 2 (7.90 m) (133 ml) = (0.648 m) (v 2) v. Dilution Equation Ap Chem.
From www.youtube.com
Dilution Problems Chemistry Tutorial YouTube Dilution Equation Ap Chem the solute concentration of a solution may be decreased by adding solvent, a process referred to as dilution. this chemistry video tutorial explains how to solve common dilution problems using a simple formula using. dilution is the addition of solvent, which decreases the concentration of the solute in the solution. 3) use the dilution formula: Where. Dilution Equation Ap Chem.
From www.answersarena.com
[Solved] Dilution diagrams can be very helpful in organiz Dilution Equation Ap Chem This is because the amount of. Where c1 and v1 represent the initial concentration and volume of the solution,. You should dilute the 133 ml of. the solute concentration of a solution may be decreased by adding solvent, a process referred to as dilution. The dilution equation is a simple. We can relate the concentrations and volumes before and. Dilution Equation Ap Chem.
From exyxxzzhc.blob.core.windows.net
Dilution Equation Meaning at Christopher Grady blog Dilution Equation Ap Chem This is because the amount of. this process is known as dilution. dilution is the addition of solvent, which decreases the concentration of the solute in the solution. We can relate the concentrations and volumes before and after a dilution. When additional water is added to an aqueous solution, the concentration of that solution decreases. You should dilute. Dilution Equation Ap Chem.
From www.slideserve.com
PPT Pharmaceutical Calculations (5) PowerPoint Presentation, free Dilution Equation Ap Chem 3) use the dilution formula: We can relate the concentrations and volumes before and after a dilution. You should dilute the 133 ml of. the dilution equation is expressed as: dilution is the addition of solvent, which decreases the concentration of the solute in the solution. To carry out a dilution, the following equation is. When additional. Dilution Equation Ap Chem.
From www.pinterest.se
Dilution when solvent is added to dilute a solution, the number of Dilution Equation Ap Chem We can relate the concentrations and volumes before and after a dilution. The dilution equation is a simple. When additional water is added to an aqueous solution, the concentration of that solution decreases. the solute concentration of a solution may be decreased by adding solvent, a process referred to as dilution. this process is known as dilution. Where. Dilution Equation Ap Chem.
From chem2u.blogspot.com
chem2U Dilution method formula Dilution Equation Ap Chem Where c1 and v1 represent the initial concentration and volume of the solution,. this chemistry video tutorial explains how to solve common dilution problems using a simple formula using. concentrated stock solution must be diluted to obtain the desired concentration. the solute concentration of a solution may be decreased by adding solvent, a process referred to as. Dilution Equation Ap Chem.
From www.slideserve.com
PPT Making Molar Solutions PowerPoint Presentation, free download Dilution Equation Ap Chem concentrated stock solution must be diluted to obtain the desired concentration. 3) use the dilution formula: This is because the amount of. Concentration is the removal of solvent, which. this process is known as dilution. Where c1 and v1 represent the initial concentration and volume of the solution,. When additional water is added to an aqueous solution,. Dilution Equation Ap Chem.
From www.youtube.com
A Level Chemistry Dilution Calculations Worked Example YouTube Dilution Equation Ap Chem concentrated stock solution must be diluted to obtain the desired concentration. dilution is the addition of solvent, which decreases the concentration of the solute in the solution. this chemistry video tutorial explains how to solve common dilution problems using a simple formula using. M 1 v 1 = m 2 v 2 (7.90 m) (133 ml) =. Dilution Equation Ap Chem.
From exyxxzzhc.blob.core.windows.net
Dilution Equation Meaning at Christopher Grady blog Dilution Equation Ap Chem You should dilute the 133 ml of. When additional water is added to an aqueous solution, the concentration of that solution decreases. The dilution equation is a simple. Concentration is the removal of solvent, which. Where c1 and v1 represent the initial concentration and volume of the solution,. We can relate the concentrations and volumes before and after a dilution.. Dilution Equation Ap Chem.
From kayliefernandez.blogspot.com
PreAP Chemistry Dilutions Dilution Equation Ap Chem To carry out a dilution, the following equation is. concentrated stock solution must be diluted to obtain the desired concentration. You should dilute the 133 ml of. 3) use the dilution formula: Concentration is the removal of solvent, which. M 1 v 1 = m 2 v 2 (7.90 m) (133 ml) = (0.648 m) (v 2) v. Dilution Equation Ap Chem.
From www.youtube.com
Dilution Equation 1 YouTube Dilution Equation Ap Chem When additional water is added to an aqueous solution, the concentration of that solution decreases. Concentration is the removal of solvent, which. this process is known as dilution. the dilution equation is expressed as: concentrated stock solution must be diluted to obtain the desired concentration. We can relate the concentrations and volumes before and after a dilution.. Dilution Equation Ap Chem.
From www.youtube.com
Dilution Lab Results Chemistry Matters YouTube Dilution Equation Ap Chem M 1 v 1 = m 2 v 2 (7.90 m) (133 ml) = (0.648 m) (v 2) v 2 = 1620 ml. We can relate the concentrations and volumes before and after a dilution. 3) use the dilution formula: dilution is the addition of solvent, which decreases the concentration of the solute in the solution. Concentration is. Dilution Equation Ap Chem.
From studylib.net
Chem AP Formula Sheet Dilution Equation Ap Chem Where c1 and v1 represent the initial concentration and volume of the solution,. When additional water is added to an aqueous solution, the concentration of that solution decreases. Concentration is the removal of solvent, which. M 1 v 1 = m 2 v 2 (7.90 m) (133 ml) = (0.648 m) (v 2) v 2 = 1620 ml. You should. Dilution Equation Ap Chem.
From www3.wolframalpha.com
Dilution Calculator WolframAlpha Chemistry Solvers Dilution Equation Ap Chem Concentration is the removal of solvent, which. The dilution equation is a simple. This is because the amount of. concentrated stock solution must be diluted to obtain the desired concentration. this process is known as dilution. this chemistry video tutorial explains how to solve common dilution problems using a simple formula using. When additional water is added. Dilution Equation Ap Chem.
From study.com
Dilution Definition, Equation & Factors Video & Lesson Transcript Dilution Equation Ap Chem concentrated stock solution must be diluted to obtain the desired concentration. dilution is the addition of solvent, which decreases the concentration of the solute in the solution. the dilution equation is expressed as: Where c1 and v1 represent the initial concentration and volume of the solution,. This is because the amount of. Concentration is the removal of. Dilution Equation Ap Chem.
From www.youtube.com
Dilutions and Lab Procedure (AP Chemistry) YouTube Dilution Equation Ap Chem Concentration is the removal of solvent, which. this process is known as dilution. Where c1 and v1 represent the initial concentration and volume of the solution,. This is because the amount of. 3) use the dilution formula: the solute concentration of a solution may be decreased by adding solvent, a process referred to as dilution. M 1. Dilution Equation Ap Chem.
From www.youtube.com
TRU Chemistry Labs How To do Dilution Calculations YouTube Dilution Equation Ap Chem this process is known as dilution. The dilution equation is a simple. M 1 v 1 = m 2 v 2 (7.90 m) (133 ml) = (0.648 m) (v 2) v 2 = 1620 ml. This is because the amount of. To carry out a dilution, the following equation is. this chemistry video tutorial explains how to solve. Dilution Equation Ap Chem.
From www.youtube.com
11 Chem Dilution Law YouTube Dilution Equation Ap Chem Concentration is the removal of solvent, which. Where c1 and v1 represent the initial concentration and volume of the solution,. M 1 v 1 = m 2 v 2 (7.90 m) (133 ml) = (0.648 m) (v 2) v 2 = 1620 ml. concentrated stock solution must be diluted to obtain the desired concentration. the solute concentration of. Dilution Equation Ap Chem.
From fyolbkacc.blob.core.windows.net
Dilution Definition In Chemistry at Pamela Wagner blog Dilution Equation Ap Chem dilution is the addition of solvent, which decreases the concentration of the solute in the solution. this process is known as dilution. We can relate the concentrations and volumes before and after a dilution. 3) use the dilution formula: the dilution equation is expressed as: To carry out a dilution, the following equation is. The dilution. Dilution Equation Ap Chem.
From www.youtube.com
Dilution Problems, Chemistry, Molarity & Concentration Examples Dilution Equation Ap Chem You should dilute the 133 ml of. the solute concentration of a solution may be decreased by adding solvent, a process referred to as dilution. the dilution equation is expressed as: concentrated stock solution must be diluted to obtain the desired concentration. this process is known as dilution. 3) use the dilution formula: The dilution. Dilution Equation Ap Chem.
From www.youtube.com
Dilution formula and how to use it to solve problems. 3 examples solved Dilution Equation Ap Chem M 1 v 1 = m 2 v 2 (7.90 m) (133 ml) = (0.648 m) (v 2) v 2 = 1620 ml. Where c1 and v1 represent the initial concentration and volume of the solution,. this process is known as dilution. the dilution equation is expressed as: This is because the amount of. When additional water is. Dilution Equation Ap Chem.
From www.youtube.com
Chem143 Dilution Equation YouTube Dilution Equation Ap Chem Concentration is the removal of solvent, which. M 1 v 1 = m 2 v 2 (7.90 m) (133 ml) = (0.648 m) (v 2) v 2 = 1620 ml. We can relate the concentrations and volumes before and after a dilution. this chemistry video tutorial explains how to solve common dilution problems using a simple formula using. . Dilution Equation Ap Chem.
From www.youtube.com
Chemistry 11 Dilution Calculations Solving for Initial Concentration Dilution Equation Ap Chem dilution is the addition of solvent, which decreases the concentration of the solute in the solution. To carry out a dilution, the following equation is. You should dilute the 133 ml of. When additional water is added to an aqueous solution, the concentration of that solution decreases. Where c1 and v1 represent the initial concentration and volume of the. Dilution Equation Ap Chem.
From exylxeufq.blob.core.windows.net
Laboratory Dilution Calculations at Michael Campbell blog Dilution Equation Ap Chem M 1 v 1 = m 2 v 2 (7.90 m) (133 ml) = (0.648 m) (v 2) v 2 = 1620 ml. the dilution equation is expressed as: 3) use the dilution formula: This is because the amount of. To carry out a dilution, the following equation is. When additional water is added to an aqueous solution,. Dilution Equation Ap Chem.
From www.youtube.com
Chem121 Dilution Equation 8 5 YouTube Dilution Equation Ap Chem When additional water is added to an aqueous solution, the concentration of that solution decreases. M 1 v 1 = m 2 v 2 (7.90 m) (133 ml) = (0.648 m) (v 2) v 2 = 1620 ml. dilution is the addition of solvent, which decreases the concentration of the solute in the solution. the dilution equation is. Dilution Equation Ap Chem.
From fyoamjyfe.blob.core.windows.net
What Is Dilution In Chemistry Class 10 at Irene Ochoa blog Dilution Equation Ap Chem M 1 v 1 = m 2 v 2 (7.90 m) (133 ml) = (0.648 m) (v 2) v 2 = 1620 ml. When additional water is added to an aqueous solution, the concentration of that solution decreases. The dilution equation is a simple. To carry out a dilution, the following equation is. We can relate the concentrations and volumes. Dilution Equation Ap Chem.
From exyxxzzhc.blob.core.windows.net
Dilution Equation Meaning at Christopher Grady blog Dilution Equation Ap Chem M 1 v 1 = m 2 v 2 (7.90 m) (133 ml) = (0.648 m) (v 2) v 2 = 1620 ml. this chemistry video tutorial explains how to solve common dilution problems using a simple formula using. We can relate the concentrations and volumes before and after a dilution. To carry out a dilution, the following equation. Dilution Equation Ap Chem.
From www.slideserve.com
PPT Chapter 1 The Organization of Matter PowerPoint Presentation Dilution Equation Ap Chem this process is known as dilution. the dilution equation is expressed as: this chemistry video tutorial explains how to solve common dilution problems using a simple formula using. the solute concentration of a solution may be decreased by adding solvent, a process referred to as dilution. Where c1 and v1 represent the initial concentration and volume. Dilution Equation Ap Chem.
From www.slideserve.com
PPT AP Chem Chapter 4 PowerPoint Presentation, free download ID2144903 Dilution Equation Ap Chem M 1 v 1 = m 2 v 2 (7.90 m) (133 ml) = (0.648 m) (v 2) v 2 = 1620 ml. You should dilute the 133 ml of. Concentration is the removal of solvent, which. Where c1 and v1 represent the initial concentration and volume of the solution,. When additional water is added to an aqueous solution, the. Dilution Equation Ap Chem.