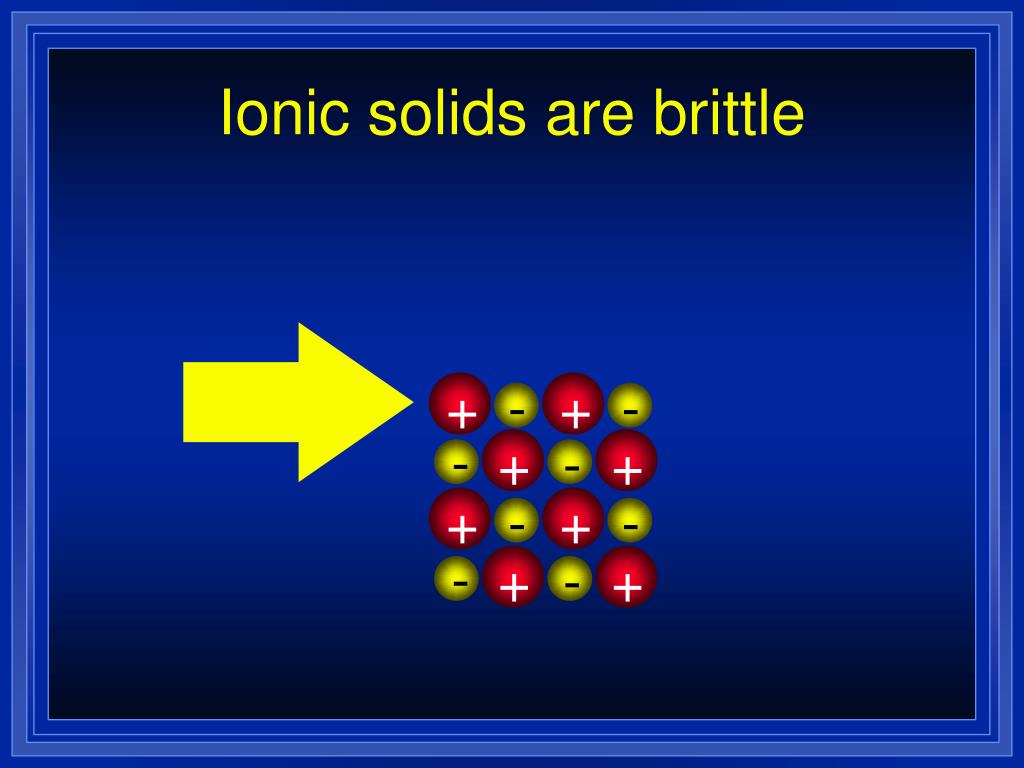
Brittle And Ionic Compounds . Covalent compounds are usually softer and more flexible than ionic compounds, which are hard and brittle. learn how ionic compounds have high melting points, hard and brittle shapes, solubility in water, and conductivity in solutions. here are the properties of ionic compounds: the hard and brittle qualities of an ionic compound are explained by its habit of forming a crystal lattice structure with. Ionic compounds are generally hard and brittle. It takes a large amount of mechanical force, such as striking a crystal with a hammer, to force one layer of ions to shift relative to its neighbor. learn how to identify ionic and covalent compounds by their properties, such as crystals, melting points, conductivity and more. See examples, diagrams, and exercises to test your understanding of ionic bonding and properties. ionic compounds are generally hard, but brittle. in summary, brittleness is not a property uniquely associated with ionic compounds. ionic compounds are electrical insulators as solids, but conductors as liquids or in water. Learn the other properties of ionic compounds, such as crystalline, hard, brittle, soluble in water and high melting points.
from www.slideserve.com
here are the properties of ionic compounds: in summary, brittleness is not a property uniquely associated with ionic compounds. Learn the other properties of ionic compounds, such as crystalline, hard, brittle, soluble in water and high melting points. Covalent compounds are usually softer and more flexible than ionic compounds, which are hard and brittle. learn how to identify ionic and covalent compounds by their properties, such as crystals, melting points, conductivity and more. It takes a large amount of mechanical force, such as striking a crystal with a hammer, to force one layer of ions to shift relative to its neighbor. Ionic compounds are generally hard and brittle. ionic compounds are generally hard, but brittle. the hard and brittle qualities of an ionic compound are explained by its habit of forming a crystal lattice structure with. See examples, diagrams, and exercises to test your understanding of ionic bonding and properties.
PPT Chapter 15 Ionic Bonding and Ionic Compounds PowerPoint
Brittle And Ionic Compounds Covalent compounds are usually softer and more flexible than ionic compounds, which are hard and brittle. learn how to identify ionic and covalent compounds by their properties, such as crystals, melting points, conductivity and more. See examples, diagrams, and exercises to test your understanding of ionic bonding and properties. It takes a large amount of mechanical force, such as striking a crystal with a hammer, to force one layer of ions to shift relative to its neighbor. Learn the other properties of ionic compounds, such as crystalline, hard, brittle, soluble in water and high melting points. the hard and brittle qualities of an ionic compound are explained by its habit of forming a crystal lattice structure with. learn how ionic compounds have high melting points, hard and brittle shapes, solubility in water, and conductivity in solutions. Covalent compounds are usually softer and more flexible than ionic compounds, which are hard and brittle. ionic compounds are generally hard, but brittle. here are the properties of ionic compounds: ionic compounds are electrical insulators as solids, but conductors as liquids or in water. Ionic compounds are generally hard and brittle. in summary, brittleness is not a property uniquely associated with ionic compounds.
From www.youtube.com
Ionic Bonding Properties of Ionic Compounds YouTube Brittle And Ionic Compounds Covalent compounds are usually softer and more flexible than ionic compounds, which are hard and brittle. learn how ionic compounds have high melting points, hard and brittle shapes, solubility in water, and conductivity in solutions. See examples, diagrams, and exercises to test your understanding of ionic bonding and properties. ionic compounds are electrical insulators as solids, but conductors. Brittle And Ionic Compounds.
From slideplayer.com
Ionic Compounds Ch.6 & ppt download Brittle And Ionic Compounds here are the properties of ionic compounds: Ionic compounds are generally hard and brittle. ionic compounds are generally hard, but brittle. ionic compounds are electrical insulators as solids, but conductors as liquids or in water. Covalent compounds are usually softer and more flexible than ionic compounds, which are hard and brittle. in summary, brittleness is not. Brittle And Ionic Compounds.
From www.slideserve.com
PPT Chapter 15 Ionic Bonding and Ionic Compounds PowerPoint Brittle And Ionic Compounds the hard and brittle qualities of an ionic compound are explained by its habit of forming a crystal lattice structure with. ionic compounds are electrical insulators as solids, but conductors as liquids or in water. Ionic compounds are generally hard and brittle. in summary, brittleness is not a property uniquely associated with ionic compounds. learn how. Brittle And Ionic Compounds.
From www.slideserve.com
PPT What are bonds? PowerPoint Presentation, free download ID5861644 Brittle And Ionic Compounds learn how to identify ionic and covalent compounds by their properties, such as crystals, melting points, conductivity and more. in summary, brittleness is not a property uniquely associated with ionic compounds. See examples, diagrams, and exercises to test your understanding of ionic bonding and properties. the hard and brittle qualities of an ionic compound are explained by. Brittle And Ionic Compounds.
From www.jagranjosh.com
What are Ionic Compounds and how they are formed? Brittle And Ionic Compounds learn how ionic compounds have high melting points, hard and brittle shapes, solubility in water, and conductivity in solutions. Ionic compounds are generally hard and brittle. See examples, diagrams, and exercises to test your understanding of ionic bonding and properties. in summary, brittleness is not a property uniquely associated with ionic compounds. learn how to identify ionic. Brittle And Ionic Compounds.
From www.slideserve.com
PPT Ions and Ionic Compounds PowerPoint Presentation, free download Brittle And Ionic Compounds learn how to identify ionic and covalent compounds by their properties, such as crystals, melting points, conductivity and more. Covalent compounds are usually softer and more flexible than ionic compounds, which are hard and brittle. It takes a large amount of mechanical force, such as striking a crystal with a hammer, to force one layer of ions to shift. Brittle And Ionic Compounds.
From www.slideserve.com
PPT BC Science Connections 9 Unit 2 The electron arrangement of Brittle And Ionic Compounds Learn the other properties of ionic compounds, such as crystalline, hard, brittle, soluble in water and high melting points. ionic compounds are electrical insulators as solids, but conductors as liquids or in water. Covalent compounds are usually softer and more flexible than ionic compounds, which are hard and brittle. ionic compounds are generally hard, but brittle. See examples,. Brittle And Ionic Compounds.
From slideplayer.com
Modern Chemistry Chapter 6 Chemical Bonding ppt download Brittle And Ionic Compounds It takes a large amount of mechanical force, such as striking a crystal with a hammer, to force one layer of ions to shift relative to its neighbor. Ionic compounds are generally hard and brittle. Covalent compounds are usually softer and more flexible than ionic compounds, which are hard and brittle. ionic compounds are electrical insulators as solids, but. Brittle And Ionic Compounds.
From www.learnatnoon.com
Physical Properties of an Ionic Compound Noon Academy Brittle And Ionic Compounds the hard and brittle qualities of an ionic compound are explained by its habit of forming a crystal lattice structure with. Covalent compounds are usually softer and more flexible than ionic compounds, which are hard and brittle. See examples, diagrams, and exercises to test your understanding of ionic bonding and properties. in summary, brittleness is not a property. Brittle And Ionic Compounds.
From slideplayer.com
Chemistry Ionic Compounds. ppt download Brittle And Ionic Compounds ionic compounds are generally hard, but brittle. It takes a large amount of mechanical force, such as striking a crystal with a hammer, to force one layer of ions to shift relative to its neighbor. learn how to identify ionic and covalent compounds by their properties, such as crystals, melting points, conductivity and more. in summary, brittleness. Brittle And Ionic Compounds.
From www.slideserve.com
PPT Properties of Substances PowerPoint Presentation, free download Brittle And Ionic Compounds See examples, diagrams, and exercises to test your understanding of ionic bonding and properties. Covalent compounds are usually softer and more flexible than ionic compounds, which are hard and brittle. learn how to identify ionic and covalent compounds by their properties, such as crystals, melting points, conductivity and more. learn how ionic compounds have high melting points, hard. Brittle And Ionic Compounds.
From www.slideserve.com
PPT Properties of ionic compounds PowerPoint Presentation ID1130121 Brittle And Ionic Compounds Covalent compounds are usually softer and more flexible than ionic compounds, which are hard and brittle. ionic compounds are generally hard, but brittle. learn how ionic compounds have high melting points, hard and brittle shapes, solubility in water, and conductivity in solutions. here are the properties of ionic compounds: Ionic compounds are generally hard and brittle. . Brittle And Ionic Compounds.
From slideplayer.com
Structure and Properties of Ionic and Covalent Compounds ppt download Brittle And Ionic Compounds here are the properties of ionic compounds: See examples, diagrams, and exercises to test your understanding of ionic bonding and properties. the hard and brittle qualities of an ionic compound are explained by its habit of forming a crystal lattice structure with. Covalent compounds are usually softer and more flexible than ionic compounds, which are hard and brittle.. Brittle And Ionic Compounds.
From www.slideshare.net
Ionic Bonding Notes Brittle And Ionic Compounds ionic compounds are electrical insulators as solids, but conductors as liquids or in water. Covalent compounds are usually softer and more flexible than ionic compounds, which are hard and brittle. See examples, diagrams, and exercises to test your understanding of ionic bonding and properties. learn how to identify ionic and covalent compounds by their properties, such as crystals,. Brittle And Ionic Compounds.
From slideplayer.com
Ionic and Metallic Bonds ppt download Brittle And Ionic Compounds Learn the other properties of ionic compounds, such as crystalline, hard, brittle, soluble in water and high melting points. learn how ionic compounds have high melting points, hard and brittle shapes, solubility in water, and conductivity in solutions. here are the properties of ionic compounds: Ionic compounds are generally hard and brittle. Covalent compounds are usually softer and. Brittle And Ionic Compounds.
From chem.libretexts.org
6.2 Comparing Ionic and Molecular Substances Chemistry LibreTexts Brittle And Ionic Compounds ionic compounds are electrical insulators as solids, but conductors as liquids or in water. in summary, brittleness is not a property uniquely associated with ionic compounds. Ionic compounds are generally hard and brittle. the hard and brittle qualities of an ionic compound are explained by its habit of forming a crystal lattice structure with. See examples, diagrams,. Brittle And Ionic Compounds.
From sciencenotes.org
Ionic Compound Properties Brittle And Ionic Compounds in summary, brittleness is not a property uniquely associated with ionic compounds. the hard and brittle qualities of an ionic compound are explained by its habit of forming a crystal lattice structure with. Covalent compounds are usually softer and more flexible than ionic compounds, which are hard and brittle. It takes a large amount of mechanical force, such. Brittle And Ionic Compounds.
From slideplayer.com
Ionic Bond Chapter 5 Section ppt download Brittle And Ionic Compounds learn how to identify ionic and covalent compounds by their properties, such as crystals, melting points, conductivity and more. learn how ionic compounds have high melting points, hard and brittle shapes, solubility in water, and conductivity in solutions. It takes a large amount of mechanical force, such as striking a crystal with a hammer, to force one layer. Brittle And Ionic Compounds.
From slideplayer.com
WRITING AND NAMING IONIC COMPOUNDS ppt download Brittle And Ionic Compounds the hard and brittle qualities of an ionic compound are explained by its habit of forming a crystal lattice structure with. ionic compounds are generally hard, but brittle. See examples, diagrams, and exercises to test your understanding of ionic bonding and properties. here are the properties of ionic compounds: in summary, brittleness is not a property. Brittle And Ionic Compounds.
From www.britannica.com
ionic bond Definition, Properties, Examples, & Facts Britannica Brittle And Ionic Compounds ionic compounds are electrical insulators as solids, but conductors as liquids or in water. Covalent compounds are usually softer and more flexible than ionic compounds, which are hard and brittle. learn how ionic compounds have high melting points, hard and brittle shapes, solubility in water, and conductivity in solutions. See examples, diagrams, and exercises to test your understanding. Brittle And Ionic Compounds.
From slideplayer.com
Ionic Bonding. ppt download Brittle And Ionic Compounds See examples, diagrams, and exercises to test your understanding of ionic bonding and properties. Covalent compounds are usually softer and more flexible than ionic compounds, which are hard and brittle. Ionic compounds are generally hard and brittle. the hard and brittle qualities of an ionic compound are explained by its habit of forming a crystal lattice structure with. It. Brittle And Ionic Compounds.
From www.shalom-education.com
Properties of Ionic Compounds GCSE Chemistry Revision Brittle And Ionic Compounds here are the properties of ionic compounds: ionic compounds are electrical insulators as solids, but conductors as liquids or in water. learn how ionic compounds have high melting points, hard and brittle shapes, solubility in water, and conductivity in solutions. the hard and brittle qualities of an ionic compound are explained by its habit of forming. Brittle And Ionic Compounds.
From ibstudy.net
4.1 Ionic bonding and structure Wang's website Brittle And Ionic Compounds Ionic compounds are generally hard and brittle. in summary, brittleness is not a property uniquely associated with ionic compounds. See examples, diagrams, and exercises to test your understanding of ionic bonding and properties. the hard and brittle qualities of an ionic compound are explained by its habit of forming a crystal lattice structure with. Covalent compounds are usually. Brittle And Ionic Compounds.
From www.youtube.com
Why ionic solids are brittle? YouTube Brittle And Ionic Compounds Ionic compounds are generally hard and brittle. learn how ionic compounds have high melting points, hard and brittle shapes, solubility in water, and conductivity in solutions. ionic compounds are electrical insulators as solids, but conductors as liquids or in water. See examples, diagrams, and exercises to test your understanding of ionic bonding and properties. Covalent compounds are usually. Brittle And Ionic Compounds.
From slideplayer.com
Ionic Bonding/Naming. ppt download Brittle And Ionic Compounds Covalent compounds are usually softer and more flexible than ionic compounds, which are hard and brittle. It takes a large amount of mechanical force, such as striking a crystal with a hammer, to force one layer of ions to shift relative to its neighbor. learn how ionic compounds have high melting points, hard and brittle shapes, solubility in water,. Brittle And Ionic Compounds.
From slidetodoc.com
TYPES OF CHEMICAL BONDS IONIC BONDS COVALENT BONDS Brittle And Ionic Compounds See examples, diagrams, and exercises to test your understanding of ionic bonding and properties. ionic compounds are generally hard, but brittle. Covalent compounds are usually softer and more flexible than ionic compounds, which are hard and brittle. learn how ionic compounds have high melting points, hard and brittle shapes, solubility in water, and conductivity in solutions. learn. Brittle And Ionic Compounds.
From slidetodoc.com
Chemistry Lesson 1 Ionic Compounds Ionic Bonds l Brittle And Ionic Compounds ionic compounds are electrical insulators as solids, but conductors as liquids or in water. learn how ionic compounds have high melting points, hard and brittle shapes, solubility in water, and conductivity in solutions. in summary, brittleness is not a property uniquely associated with ionic compounds. Ionic compounds are generally hard and brittle. ionic compounds are generally. Brittle And Ionic Compounds.
From www.linstitute.net
Edexcel IGCSE Chemistry 复习笔记 1.6 5 Ionic compounds Bonds, Structure Brittle And Ionic Compounds Covalent compounds are usually softer and more flexible than ionic compounds, which are hard and brittle. in summary, brittleness is not a property uniquely associated with ionic compounds. Ionic compounds are generally hard and brittle. learn how ionic compounds have high melting points, hard and brittle shapes, solubility in water, and conductivity in solutions. See examples, diagrams, and. Brittle And Ionic Compounds.
From www.nagwa.com
Question Video Understanding How to Represent the Crystal Structure of Brittle And Ionic Compounds learn how to identify ionic and covalent compounds by their properties, such as crystals, melting points, conductivity and more. ionic compounds are generally hard, but brittle. It takes a large amount of mechanical force, such as striking a crystal with a hammer, to force one layer of ions to shift relative to its neighbor. the hard and. Brittle And Ionic Compounds.
From slideplayer.com
Ionic and Covalent Compounds ppt download Brittle And Ionic Compounds the hard and brittle qualities of an ionic compound are explained by its habit of forming a crystal lattice structure with. Ionic compounds are generally hard and brittle. Learn the other properties of ionic compounds, such as crystalline, hard, brittle, soluble in water and high melting points. here are the properties of ionic compounds: ionic compounds are. Brittle And Ionic Compounds.
From www.slideserve.com
PPT Chapter 15 Ionic Bonding and Ionic Compounds PowerPoint Brittle And Ionic Compounds Learn the other properties of ionic compounds, such as crystalline, hard, brittle, soluble in water and high melting points. the hard and brittle qualities of an ionic compound are explained by its habit of forming a crystal lattice structure with. Covalent compounds are usually softer and more flexible than ionic compounds, which are hard and brittle. Ionic compounds are. Brittle And Ionic Compounds.
From www.slideserve.com
PPT Properties of ionic compounds PowerPoint Presentation, free Brittle And Ionic Compounds ionic compounds are electrical insulators as solids, but conductors as liquids or in water. in summary, brittleness is not a property uniquely associated with ionic compounds. here are the properties of ionic compounds: Ionic compounds are generally hard and brittle. learn how ionic compounds have high melting points, hard and brittle shapes, solubility in water, and. Brittle And Ionic Compounds.
From slideplayer.com
Ionic Solids 201 Chemistry. ppt download Brittle And Ionic Compounds See examples, diagrams, and exercises to test your understanding of ionic bonding and properties. Ionic compounds are generally hard and brittle. ionic compounds are generally hard, but brittle. learn how ionic compounds have high melting points, hard and brittle shapes, solubility in water, and conductivity in solutions. here are the properties of ionic compounds: in summary,. Brittle And Ionic Compounds.
From slideplayer.com
Ionic Bonds Chapter 5 Section ppt download Brittle And Ionic Compounds ionic compounds are generally hard, but brittle. Covalent compounds are usually softer and more flexible than ionic compounds, which are hard and brittle. Learn the other properties of ionic compounds, such as crystalline, hard, brittle, soluble in water and high melting points. learn how to identify ionic and covalent compounds by their properties, such as crystals, melting points,. Brittle And Ionic Compounds.
From www.slideserve.com
PPT Ionic and Covalent bonds PowerPoint Presentation, free download Brittle And Ionic Compounds the hard and brittle qualities of an ionic compound are explained by its habit of forming a crystal lattice structure with. here are the properties of ionic compounds: Covalent compounds are usually softer and more flexible than ionic compounds, which are hard and brittle. Ionic compounds are generally hard and brittle. Learn the other properties of ionic compounds,. Brittle And Ionic Compounds.